N-(4-Acetyl-3-methyl-1-phenyl-1H-pyrazol-5-yl)-N-methyl-2-(2-methyl-4-oxo-3,4-dihydroquinazolin-3-yl)benzamide
- PMID: 24098258
- PMCID: PMC3790439
- DOI: 10.1107/S1600536813025683
N-(4-Acetyl-3-methyl-1-phenyl-1H-pyrazol-5-yl)-N-methyl-2-(2-methyl-4-oxo-3,4-dihydroquinazolin-3-yl)benzamide
Abstract
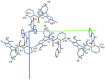
In the title compound, C29H25N5O3, the dihedral angle between the benzene ring and the pendant quinazoline ring system (r.m.s. deviation = 0.036Å) is 87.60 (17)°. The equivalent angle between the pyrazole ring and the phenyl group is 70.0 (2)°. The dihedral angle between the benzene and pyrazole rings is 30.7 (2)° and overall, the mol-ecular conformation approximates to a Z shape. A short intra-molecular C-H⋯O contact occurs. In the crystal, the mol-ecules are linked by Cπ-H⋯O-type hydrogen bonds and aromatic π-π stacking inter-actions [centroid-centroid distance = 3.860 (3) Å], generating a three-dimensional network.
Figures

References
-
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst. 27, 435.
-
- Duke, N. E. C. & Codding, P. W. (1993). Acta Cryst. B49, 719–726. - PubMed
-
- Enraf–Nonius (1994). CAD-4 EXPRESS Enraf–Nonius, Delft, The Netherlands.
-
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
-
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
