Validation of DTI tractography-based measures of primary motor area connectivity in the squirrel monkey brain
- PMID: 24098365
- PMCID: PMC3788067
- DOI: 10.1371/journal.pone.0075065
Validation of DTI tractography-based measures of primary motor area connectivity in the squirrel monkey brain
Abstract
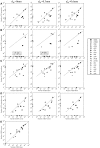
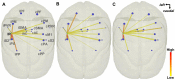
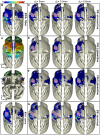
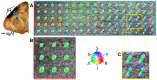
Diffusion tensor imaging (DTI) tractography provides noninvasive measures of structural cortico-cortical connectivity of the brain. However, the agreement between DTI-tractography-based measures and histological 'ground truth' has not been quantified. In this study, we reconstructed the 3D density distribution maps (DDM) of fibers labeled with an anatomical tracer, biotinylated dextran amine (BDA), as well as DTI tractography-derived streamlines connecting the primary motor (M1) cortex to other cortical regions in the squirrel monkey brain. We evaluated the agreement in M1-cortical connectivity between the fibers labeled in the brain tissue and DTI streamlines on a regional and voxel-by-voxel basis. We found that DTI tractography is capable of providing inter-regional connectivity comparable to the neuroanatomical connectivity, but is less reliable measuring voxel-to-voxel variations within regions.
Conflict of interest statement
Figures




References
-
- Stejskal EO, Tanner JE (1965) Spin diffusion measurements: Spin echoes in the presence of a time-dependent field gradient. Journal of Chemical Physics 42: 288–292.
-
- Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR in Biomedicine 15: 435–455. - PubMed
-
- Mori S, Zhang JY (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. - PubMed
-
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000) In vivo fiber tractography using DT-MRI data. Magnetic Resonance in Medicine 44: 625–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- EY08126/EY/NEI NIH HHS/United States
- R01 DA028303/DA/NIDA NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- HD15052/HD/NICHD NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- DK58404/DK/NIDDK NIH HHS/United States
- 1S10 RR 17789/RR/NCRR NIH HHS/United States
- R01-NS58639/NS/NINDS NIH HHS/United States
- R01 NS058639/NS/NINDS NIH HHS/United States
- DK59637/DK/NIDDK NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01-DA028303/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

