Differential binding of IgG and IgA to mucus of the female reproductive tract
- PMID: 24098437
- PMCID: PMC3788792
- DOI: 10.1371/journal.pone.0076176
Differential binding of IgG and IgA to mucus of the female reproductive tract
Abstract
Cells of the endocervix are responsible for the secretion of mucins, which provide an additional layer of protection to the female reproductive tract (FRT). This barrier is likely fortified with IgA as has previously been shown in the gastrointestinal tract and lungs of mice. Mucus associated IgA can facilitate clearance of bacteria. While a similar function for IgG has been proposed, an association with mucus has not yet been demonstrated. Here we find that IgA and IgG are differentially associated with the different types of mucus of the FRT. We observed that while both IgA and IgG are stably associated with cervical mucus, only IgG is associated with cervicovaginal mucus. These findings reveal that antibodies can bind tightly to mucus, where they can play a significant role in the fortification of the mucus barriers of the FRT. It may be possible to harness this interaction in the development of vaccines designed to protect the FRT mucosal barriers from sexually transmitted diseases such as HIV.
Conflict of interest statement
Figures

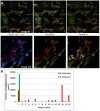
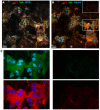


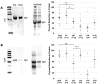
References
-
- Linford E (1974) Cervical mucus: an agent or a barrier to conception? J Reprod Fertil 37: 239–250. - PubMed
-
- Andersch-Bjorkman Y, Thomsson KA, Holmen Larsson JM, Ekerhovd E, Hansson GC (2007) Large scale identification of proteins, mucins, and their O-glycosylation in the endocervical mucus during the menstrual cycle. Mol Cell Proteomics 6: 708–716. - PubMed
-
- Gipson IK (2001) Mucins of the human endocervix. Front Biosci 6: D1245–1255. - PubMed
-
- Barros C, Arguellos B, Jedlicki A, Vigil P, Herrera E (1985) Scanning Electron Microscope Study of Human Cervical Mucus. Gamete Research 12: 85–89.
-
- Bonilla-Musoles F (1983) Scanner electron microscopy of the cervical mucus. Clin Exp Obstet Gynecol 10: 151–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

