Melanotic pathology and vertical transmission of the gut commensal Elizabethkingia meningoseptica in the major malaria vector Anopheles gambiae
- PMID: 24098592
- PMCID: PMC3788111
- DOI: 10.1371/journal.pone.0077619
Melanotic pathology and vertical transmission of the gut commensal Elizabethkingia meningoseptica in the major malaria vector Anopheles gambiae
Abstract
Background: The resident gut flora is known to have significant impacts on the life history of the host organism. Endosymbiotic bacterial species in the Anopheles mosquito gut are potent modulators of sexual development of the malaria parasite, Plasmodium, and thus proposed as potential control agents of malaria transmission.
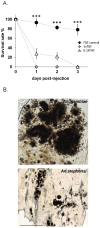
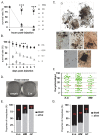
Results: Here we report a melanotic pathology in the major African malaria vector Anopheles gambiae, caused by the dominant mosquito endosymbiont Elizabethkingiameningoseptica. Transfer of melanised tissues into the haemolymph of healthy adult mosquitoes or direct haemolymph inoculation with isolated E. meningoseptica bacteria were the only means for transmission and de novo formation of melanotic lesions, specifically in the fat body tissues of recipient individuals. We show that E. meningoseptica can be vertically transmitted from eggs to larvae and that E. meningoseptica-mono-associated mosquitoes display significant mortality, which is further enhanced upon Plasmodium infection, suggesting a synergistic impact of E. meningoseptica and Plasmodium on mosquito survival.
Conclusion: The high pathogenicity and permanent association of E. meningoseptica with An. Gambiae through vertical transmission constitute attractive characteristics towards the potential design of novel mosquito/malaria biocontrol strategies.
Conflict of interest statement
Figures

References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307: 1915-1920. doi:10.1126/science.1104816. PubMed: 15790844. - DOI - PubMed
-
- Ley RE, Peterson DA, Gordon JI (2006) Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124: 837-848. doi:10.1016/j.cell.2006.02.017. PubMed: 16497592. - DOI - PubMed
-
- Pumpuni CB, Beier MS, Nataro JP, Guers LD, Davis JR (1993) Plasmodium falciparum: inhibition of sporogonic development in Anopheles stephensi by gram-negative bacteria. Exp Parasitol 77: 195-199. doi:10.1006/expr.1993.1076. PubMed: 8375488. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

