Disruption of sarcoendoplasmic reticulum calcium ATPase function in Drosophila leads to cardiac dysfunction
- PMID: 24098595
- PMCID: PMC3789689
- DOI: 10.1371/journal.pone.0077785
Disruption of sarcoendoplasmic reticulum calcium ATPase function in Drosophila leads to cardiac dysfunction
Abstract
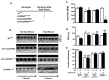
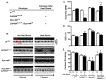
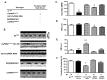
Abnormal sarcoendoplasmic reticulum Calcium ATPase (SERCA) function has been associated with poor cardiac function in humans. While modifiers of SERCA function have been identified and studied using animal models, further investigation has been limited by the absence of a model system that is amenable to large-scale genetic screens. Drosophila melanogaster is an ideal model system for the investigation of SERCA function due to the significant homology to human SERCA and the availability of versatile genetic screening tools. To further the use of Drosophila as a model for examining the role of SERCA in cardiac function, we examined cardiac function in adult flies. Using optical coherence tomography (OCT) imaging in awake, adult Drosophila, we have been able to characterize cardiac chamber dimensions in flies with disrupted in Drosophila SERCA (CaP60A). We found that the best studied CaP60A mutant, the conditional paralytic mutant CaP60A(kum170), develops marked bradycardia and chamber enlargement that is closely linked to the onset of paralysis and dependent on extra cardiac CaP60A. In contrast to prior work, we show that disruption of CaP60A in a cardiac specific manner results in cardiac dilation and dysfunction rather than alteration in heart rate. In addition, the co-expression of a calcium release channel mutation with CaP60A (kum170) is sufficient to rescue the cardiac phenotype but not paralysis. Finally, we show that CaP60A overexpression is able to rescue cardiac function in a model of Drosophila cardiac dysfunction similar to what is observed in mammals. Thus, we present a cardiac phenotype associated with Drosophila SERCA dysfunction that would serve as additional phenotyping for further large-scale genetic screens for novel modifiers of SERCA function.
Conflict of interest statement
Figures


References
-
- Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL et al. (1998) Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res 83: 889-897. doi: 10.1161/01.RES.83.9.889. PubMed: 9797337. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

