Increased protein stability of CDKN1C causes a gain-of-function phenotype in patients with IMAGe syndrome
- PMID: 24098681
- PMCID: PMC3787065
- DOI: 10.1371/journal.pone.0075137
Increased protein stability of CDKN1C causes a gain-of-function phenotype in patients with IMAGe syndrome
Abstract
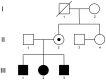
Mutations in the proliferating cell nuclear antigen (PCNA)-binding domain of the CDKN1C gene were recently identified in patients with IMAGe syndrome. However, loss of PCNA binding and suppression of CDKN1C monoubiquitination by IMAGe-associated mutations hardly explain the reduced-growth phenotype characteristic of IMAGe syndrome. We demonstrate here that IMAGe-associated mutations in the CDKN1C gene dramatically increased the protein stability. We identified a novel heterozygous mutation, c.815T>G (p.Ile272Ser), in the CDKN1C gene in three siblings manifesting clinical symptoms associated with IMAGe syndrome and their mother (unaffected carrier). PCNA binding to CDKN1C was disrupted in the case of p.Ile272Ser, and for two other IMAGe-associated mutations, p.Asp274Asn and p.Phe276Val. Intriguingly, the IMAGe-associated mutant CDKN1C proteins were fairly stable even in the presence of cycloheximide, whereas the wild-type protein was almost completely degraded via the proteasome pathway, as shown by the lack of degradation with addition of a proteasome inhibitor, MG132. These results thus suggested that the reduced-growth phenotype of IMAGe syndrome derives from CDKN1C gain-of-function due to IMAGe-associated mutations driving increased protein stability.
Conflict of interest statement
Figures



References
-
- Vilain E, Le Merrer M, Lecointre C, Desangles F, Kay MA, et al. (1999) IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab 84: 4335–4340. - PubMed
-
- Bergadá I, Del Rey G, Lapunzina P, Bergadá C, Fellous M, et al. (2005) Familial occurrence of the IMAGe association: additional clinical variants and a proposed mode of inheritance. J Clin Endocrinol Metab 90: 3186–3190. - PubMed
-
- Hutz JE, Krause AS, Achermann JC, Vilain E, Tauber M, et al. (2006) IMAGe association and congenital adrenal hypoplasia: no disease-causing mutations found in the ACD gene. Mol Genet Metab 88: 66–70. - PubMed
-
- Lienhardt A, Mas JC, Kalifa G, Chaussain JL, Tauber M (2002) IMAGe association: additional clinical features and evidence for recessive autosomal inheritance. Horm Res 57: 71–78. - PubMed
-
- Pedreira CC, Savarirayan R, Zacharin MR (2004) IMAGe syndrome: a complex disorder affecting growth, adrenal and gonadal function, and skeletal development. J Pediatr 144: 274–277. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

