Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells
- PMID: 24098776
- PMCID: PMC3786983
- DOI: 10.1371/journal.pone.0076157
Bone marrow mesenchymal stem cells stimulate proliferation and neuronal differentiation of retinal progenitor cells
Abstract
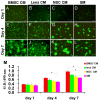
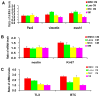
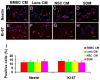
During retina development, retinal progenitor cell (RPC) proliferation and differentiation are regulated by complex inter- and intracellular interactions. Bone marrow mesenchymal stem cells (BMSCs) are reported to express a variety of cytokines and neurotrophic factors, which have powerful trophic and protective functions for neural tissue-derived cells. Here, we show that the expanded RPC cultures treated with BMSC-derived conditioned medium (CM) which was substantially enriched for bFGF and CNTF, expressed clearly increased levels of nuclear receptor TLX, an essential regulator of neural stem cell (NSC) self-renewal, as well as betacellulin (BTC), an EGF-like protein described as supporting NSC expansion. The BMSC CM- or bFGF-treated RPCs also displayed an obviously enhanced proliferation capability, while BMSC CM-derived bFGF knocked down by anti-bFGF, the effect of BMSC CM on enhancing RPC proliferation was partly reversed. Under differentiation conditions, treatment with BMSC CM or CNTF markedly favoured RPC differentiation towards retinal neurons, including Brn3a-positive retinal ganglion cells (RGCs) and rhodopsin-positive photoreceptors, and clearly diminished retinal glial cell differentiation. These findings demonstrate that BMSCs supported RPC proliferation and neuronal differentiation which may be partly mediated by BMSC CM-derived bFGF and CNTF, reveal potential limitations of RPC culture systems, and suggest a means for optimizing RPC cell fate determination in vitro.
Conflict of interest statement
Figures




References
-
- Rivolta C, Sharon D, DeAngelis MM, Dryja TP (2002) Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum Mol Genet 11: 1219-1227. doi:10.1093/hmg/11.10.1219. PubMed: 12015282. - DOI - PubMed
-
- Coles BL, Angénieux B, Inoue T, Del Rio-Tsonis K, Spence JR et al. (2004) Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A 101: 15772-15777. doi:10.1073/pnas.0401596101. PubMed: 15505221. - DOI - PMC - PubMed
-
- Strassburg S, Richardson SM, Freemont AJ, Hoyland JA (2010) Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. Regen Med 5: 701-711. doi:10.2217/rme.10.59. PubMed: 20868326. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

