The uremic toxin indoxyl sulphate enhances macrophage response to LPS
- PMID: 24098806
- PMCID: PMC3786936
- DOI: 10.1371/journal.pone.0076778
The uremic toxin indoxyl sulphate enhances macrophage response to LPS
Abstract
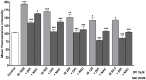
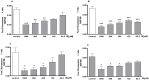
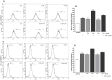
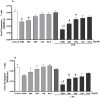
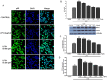
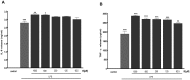
Indoxyl sulphate (IS) is a protein-bound uremic toxin that results from the metabolism of dietary tryptophan normally excreted by kidney through the proximal tubules. Thus the toxin accumulates in the blood of patients with impaired renal function such as in chronic kidney disease (CKD). High IS serum levels in patients with CKD suggest its involvement in CKD progression and in the onset of complications. Its presence in plasma is also a powerful predictor of overall and cardiovascular morbidity/mortality. IS is a well known nephrovascular toxin but very little is known regarding its effects on the immune system and in particular during inflammation. In this study we examined the effect of IS on macrophage activation in response to lipopolysaccharide from E. coli (LPS), a gram negative bacterial endotoxin associated with inflammation and septic shock. To simulate the uremic condition, J774A.1 macrophages were incubated with IS at concentrations observed in uremic patients (1000-62.5 µM) both alone and during LPS challenge. IS alone induced release of reactive oxygen species (ROS), through a mechanism involving pro- and anti-oxidant systems, and alteration in intracellular calcium homeostasis. When added to J774A.1 macrophages in presence of LPS, IS significantly increased the nitric oxide (NO) release, inducible nitric oxide synthase (iNOS) and cycloxygenase-2 (COX-2) expression. IS pre-treatment was also associated with an increase in tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) production by macrophages stimulated with LPS. Mechanistic studies revealed that IS increased LPS-induced NF-kB nuclear translocation, ROS release and altered calcium concentrations, mainly because of mitochondrial calcium overloading. Moreover also in primary mouse peritoneal macrophages IS enhances the inflammatory response to LPS increasing ROS, NO, iNOS, COX-2, TNF-α, IL-6 and NF-kB levels. This study provides evidences that IS stimulates macrophage function and enhances inflammatory reasponse associated with LPS, thus contributing to altered immune response dysfunctions observed in CKD.
Conflict of interest statement
Figures

References
-
- Vanholder R, Argiles A, Baurmeister U, Brunet P, Clark W, et al. (2001) Uremic toxicity: Present state of the art. Int J Artif Organs 24: 695–725. - PubMed
-
- Raff AC, Meyer TW, Hostetter TH (2008) New insights into uremic toxicity. Curr Opin Nephrol Hypertens 17: 560–565. - PubMed
-
- Di Micco L, Marzocco S, Sirico ML, Torraca S, Di Iorio B (2012) Does daily dialysis improve hypertension in chronic haemodialysis patients? Current Hypertension Reviews 8(4): 291–295.
-
- Niwa T, Takeda N, Tatematsu A, Maeda K (1988) Accumulation of indoxyl sulfate, an inhibitor of drug-binding, in uremic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin Chem 34: 2264–2267. - PubMed
-
- Niwa T (2001) Uremic Toxicity, Indoxyl sulfate. In: Massry SG, Glassock RJ (eds). Textbook of Nephrology. Philadelphia, PA: LippincottWilliams & Wilkins, 1269–1272.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

