Design, synthesis, and activity of a series of arylpyrid-3-ylmethanones as type I positive allosteric modulators of α7 nicotinic acetylcholine receptors
- PMID: 24098954
- PMCID: PMC3912855
- DOI: 10.1021/jm400704g
Design, synthesis, and activity of a series of arylpyrid-3-ylmethanones as type I positive allosteric modulators of α7 nicotinic acetylcholine receptors
Abstract
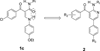
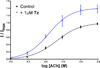
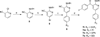
A series of novel arylpyrid-3-ylmethanones (7a-aa) were designed as modulators of α7 nicotinic acetylcholine receptors (nAChRs). The methanones were found to be type I positive allosteric modulators (PAMs) of human α7 nAChRs expressed in Xenopus ooctyes. Structure-activity relationship (SAR) studies resulted in the identification of compound 7v as a potent and efficacious type I PAM with maximum modulation of a nicotine EC5 response of 1200% and EC50 = 0.18 μM. Compound 7z was active in reversing the effect of scopolamine in the novel object recognition (NOR) paradigm with a minimum effective ip dose of 1.0 mg/kg (2.7 μmol/kg). This effect was blocked by the selective α7 nAChR antagonist methyllycaconitine (MLA). These compounds are potent type I positive allosteric modulators of α7 nAChRs that may have therapeutic value in restoring impaired sensory gating and cognitive deficits in schizophrenia and Alzheimer's disease.
Figures









References
-
- Gotti C, Riganti L, Vailati S, Clementi F. Brain Neuronal Nicotinic Receptors as New Targets for Drug Discovery. Curr. Pharm. Des. 2006;12:407–428. - PubMed
- Briggs CA, Grønlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, Kerr P, Anderson DJ, Malysz J, Dyhring T, Olsen GM, Peters D, Bunnelle WH, Gopalakrishnan M. Role of Channel Activation in Cognitive Enhancement Mediated by α7 Nicotinic Acetylcholine Receptors. Br. Journal Pharm. 2009;158:1486–1494. - PMC - PubMed
- Leiser SC, Bowlby MR, Comery TA, Dunlop J. A Cog in Cognition: How the α7 Nicotinic Acetylcholine Receptor is Geared Toward Improving Cognitive Deficits. Pharmacol. Ther. 2009;122:302–311. - PubMed
- Jensen AA, Frlund B, Liljefors T, Krogsgaard-Larsen P. Neuronal Nicotinic Acetylcholine Receptors: Structural Revelations Target Identifications Therapeutic Inspirations. J. Med. Chem. 2005;48:4705–4745. - PubMed
- Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P. Nicotinic Receptors: Allosteric Transitions and Therapeutic Targets in the Nervous System. Nat. Rev. Drug Discovery. 2009;8:733–750. - PubMed
- Hajós M, Rogers BN. Targeting α7 Nicotinic Acetylcholine Receptors in the Treatment of Schizophrenia. Curr. Pharm. Des. 2010;16:538–554. - PubMed
- Olincy A, Freedman R. Nicotinic Mechanisms in the Treatment of Psychotic Disorders: A Focus on the α7 Nicotinic Receptor. In: Meyer MA, Gross G, editors. Novel Antischizophrenia Treatments in Handbook of Experimental Pharmacology 213. Heidelberg: Springer-Verlag; 2012. pp. 211–232. - PMC - PubMed
-
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and Functional Diversity of Native Brain Neuronal Nicotinic Receptors. Biochem. Pharmacol. 2009;78:703–711. - PubMed
-
- Haydar SN, Dunlop J. Neuronal Nicotinic Acetylcholine Receptors – Targets for the Development of Drugs to Treat Cognitive Impairment Associated with Schizophrenia and Alzheimer’s Disease. Curr. Top. Med. Chem. 2010;10:144–152. - PubMed
-
- Hogg RC, Bertrand D. Partial agonists as therapeutic agents at neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2007;73:459–468. - PubMed
-
- Faghih R, Gopalakrishnan M, Briggs CA. Allosteric Modulators of the α7 Nicotinic Acetylcholine Receptor. J. Med. Chem. 2008;51:701–712. - PubMed
- Grønlien JH, Håkerud M, Ween H, Thorin-Hagene K, Briggs CA, Gopalakrishnan M, Malysz J. Distinct Profiles of α7 nAChR Positive Allosteric Modulation Revealed by Structurally Diverse Chemotypes. Mol. Pharmacol. 2007;72:715–724. - PubMed
- Malysz J, Gronlien JH, Timmermann DB, Hakerud M, Thorin-Hagene K, Ween H, Trumbull JD, Xiong Y, Briggs CA, Ahring PK, Dyhring T. Gopalakrishnan. Evaluation of alpha7 Nicotinic Acetylcholine Receptor Agonists and Positive Allosteric Modulators Using the Parallel Oocyte Electrophysiology Test Station. Assay Drug Dev. Technol. 2009;7:374–390. - PubMed
- Dunlop J, Lock T, Jow B, Sitzia F, Grauer S, Jow F, Kramer A, Bowlby MR, Randall A, Kowal D, Gilbert A, Comery TA, LaRocque J, Soloveva V, Brown J, Roncarati R. Old and New Pharmacology: Positive Allosteric Modulation of the alpha7 Nicotinic Acetylcholine Receptor by the 5-Hydroxytryptamine2B/C Receptor Antagonist SB-206553 (3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b′]dipyrrole-1(2H)-carboxamide) J. Pharmacol. Exp. Ther. 2009;328:766–776. - PubMed
- Dinklo T, Shaban H, Thuring JW, Lavreysen H, Stevens KE, Zheng L, Mackie C, Grantham C, Vandenberk I, Meulders G, Peeters L, Verachtert H, De Prins E, Lesage ASJ. Characterization of 2-[[4-Fluoro-3-(trifluoromethyl)phenyl]amino]-4-(4-pyridinyl)-5-thiazolemethanol (JNJ-1930942), a Novel Positive Allosteric Modulator of the α7 Nicotinic Acetylcholine Receptor. J. Pharmacol. Exp. Ther. 2011;336:560–574. - PubMed
- Williams DK, Wang J, Papke RL. Positive Allosteric Modulators as an Approach to Nicotinic Acetylcholine Receptor-Targeted Therapeutics: Advantages and Limitations. Biochem. Pharmacol. 2011;82:915–930. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

