Biochemical and structural insights into intramembrane metalloprotease mechanisms
- PMID: 24099006
- PMCID: PMC3793210
- DOI: 10.1016/j.bbamem.2013.03.032
Biochemical and structural insights into intramembrane metalloprotease mechanisms
Abstract
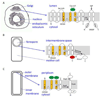
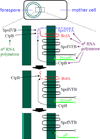
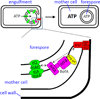
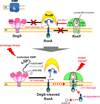
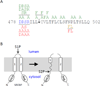
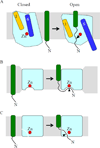
Intramembrane metalloproteases are nearly ubiquitous in living organisms and they function in diverse processes ranging from cholesterol homeostasis and the unfolded protein response in humans to sporulation, stress responses, and virulence of bacteria. Understanding how these enzymes function in membranes is a challenge of fundamental interest with potential applications if modulators can be devised. Progress is described toward a mechanistic understanding, based primarily on molecular genetic and biochemical studies of human S2P and bacterial SpoIVFB and RseP, and on the structure of the membrane domain of an archaeal enzyme. Conserved features of the enzymes appear to include transmembrane helices and loops around the active site zinc ion, which may be near the membrane surface. Extramembrane domains such as PDZ (PSD-95, DLG, ZO-1) or CBS (cystathionine-β-synthase) domains govern substrate access to the active site, but several different mechanisms of access and cleavage site selection can be envisioned, which might differ depending on the substrate and the enzyme. More work is needed to distinguish between these mechanisms, both for enzymes that have been relatively well-studied, and for enzymes lacking PDZ and CBS domains, which have not been studied. This article is part of a Special Issue entitled: Intramembrane Proteases.
Keywords: (PSD-95, DLG, ZO-1); (cystathionine-β-synthase); CBS; CBS domain; GFP; IMMP(s); IP(s); Intramembrane metalloprotease; OMP(s); PDZ; PDZ domain; RNA polymerase; RNAP; RseP; S1P; S2P; SCAP; SREBP(s); SREBP-cleavage-activating protein; SpoIVFB; TMS(s); green fluorescent protein; intramembrane metalloprotease(s); intramembrane protease(s); outer membrane protein(s); site-1 protease; site-2 protease; sterol-regulatory element-binding protein(s); transmembrane segment(s).
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures


References
-
- Rawson R, Zelenski N, Nijhawan D, Ye J, Sakai J, Hasan M, Chang T, Brown M, Goldstein J. Complementation cloning of SP2, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Mol. Cell. 1997;1:47–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

