Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update
- PMID: 24099077
- PMCID: PMC4086638
- DOI: 10.5858/arpa.2013-0953-SA
Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update
Abstract
Purpose: To update the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guideline recommendations for human epidermal growth factor receptor 2 (HER2) testing in breast cancer to improve the accuracy of HER2 testing and its utility as a predictive marker in invasive breast cancer.
Methods: ASCO/CAP convened an Update Committee that included coauthors of the 2007 guideline to conduct a systematic literature review and update recommendations for optimal HER2 testing.
Results: The Update Committee identified criteria and areas requiring clarification to improve the accuracy of HER2 testing by immunohistochemistry (IHC) or in situ hybridization (ISH). The guideline was reviewed and approved by both organizations.
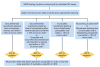
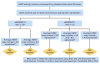
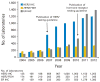
Recommendations: The Update Committee recommends that HER2 status (HER2 negative or positive) be determined in all patients with invasive (early stage or recurrence) breast cancer on the basis of one or more HER2 test results (negative, equivocal, or positive). Testing criteria define HER2-positive status when (on observing within an area of tumor that amounts to >10% of contiguous and homogeneous tumor cells) there is evidence of protein overexpression (IHC) or gene amplification (HER2 copy number or HER2/CEP17 ratio by ISH based on counting at least 20 cells within the area). If results are equivocal (revised criteria), reflex testing should be performed using an alternative assay (IHC or ISH). Repeat testing should be considered if results seem discordant with other histopathologic findings. Laboratories should demonstrate high concordance with a validated HER2 test on a sufficiently large and representative set of specimens. Testing must be performed in a laboratory accredited by CAP or another accrediting entity. The Update Committee urges providers and health systems to cooperate to ensure the highest quality testing.
Figures

Comment in
-
In Reply.Arch Pathol Lab Med. 2016 Aug;140(8):741. doi: 10.5858/arpa.2016-0105-LE. Arch Pathol Lab Med. 2016. PMID: 27472229 No abstract available.
References
-
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. - PubMed
-
- Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. - PubMed
-
- Hammond EH, Wolff AC, Hayes DF, et al. Reply to Sauter G. et al. J Clin Oncol. 2009;27:e153–e154. author reply e155-e157. - PubMed
-
- Hammond ME, Hayes DF, Wolff AC. Clinical notice for American Society of Clinical Oncology-College of American Pathologists guideline recommendations on ER/PgR and HER2 testing in breast cancer. J Clin Oncol. 2011;29:e458. - PubMed
-
- Wolff AC, Hammond ME, Hayes DF. Re: Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst. 2012;104:957–958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
