Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease
- PMID: 24099797
- PMCID: PMC3840081
- DOI: 10.1016/j.bcp.2013.09.024
Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease
Abstract
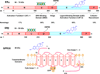
Cardiovascular disease (CVD) is less common in premenopausal women than men of the same age or postmenopausal women, suggesting vascular benefits of estrogen. Estrogen activates estrogen receptors ERα, ERβ and GPR30 in endothelium and vascular smooth muscle (VSM), which trigger downstream signaling pathways and lead to genomic and non-genomic vascular effects such as vasodilation, decreased VSM contraction and growth and reduced vascular remodeling. However, randomized clinical trials (RCTs), such as the Women's Health Initiative (WHI) and Heart and Estrogen/progestin Replacement Study (HERS), have shown little vascular benefits and even adverse events with menopausal hormone therapy (MHT), likely due to factors related to the MHT used, ER profile, and RCT design. Some MHT forms, dose, combinations or route of administration may have inadequate vascular effects. Age-related changes in ER amount, distribution, integrity and post-ER signaling could alter the vascular response to MHT. The subject's age, preexisting CVD, and hormone environment could also reduce the effects of MHT. Further evaluation of natural and synthetic estrogens, phytoestrogens, and selective estrogen-receptor modulators (SERMs), and the design of appropriate MHT combinations, dose, route and 'timing' could improve the effectiveness of conventional MHT and provide alternative therapies in the peri-menopausal period. Targeting ER using specific ER agonists, localized MHT delivery, and activation of specific post-ER signaling pathways could counter age-related changes in ER. Examination of the hormone environment and conditions associated with hormone imbalance such as polycystic ovary syndrome may reveal the causes of abnormal hormone-receptor interactions. Consideration of these factors in new RCTs such as the Kronos Early Estrogen Prevention Study (KEEPS) could enhance the vascular benefits of estrogen in postmenopausal CVD.
Keywords: 17β-estradiol; 27-hydroxycholesterol; 27HC; Akt; AngII; C-reactive protein; CEE; CRP; CVD; E2; EC; ECM; ELITE; ER; Early versus Late Intervention Trial with Estradiol; Endothelium; Extracellular matrix; FMD; G protein-coupled receptor 30; GPR30; HERS; HSP90; Hypertension; IL-6; KEEPS; Kronos early estrogen prevention study; MAPK; MHT; MI; MMP; MPA; NHS; NO; Nurses’ Health Study; OVX; P4; PCOS; PI(3)K; Post-MW; Pre-MW; Progesterone; RCT; SHR; Sex hormones; T; TMF-α; TXA2; Testosterone; VSM; VSM cell; VSMC; VTE; Vascular smooth muscle; WHI; Women's Health Initiative; angiotensin II; cardiovascular disease; conjugated equine estrogen; eNOS; endothelial cell; endothelial nitric oxide synthase; estrogen receptor; extracellular matrix; flow mediated dilation; heart and estrogen/progestin replacement study; heat shock protein-90; interleukin-6; matrix metalloproteinase; medroxyprogesterone acetate; menopausal hormone therapy; mitogen-activated protein kinase; myocardial infarction; nitric oxide; ovariectomized; phosphatidylinositol 3-kinase; polycystic ovary syndrome; postmenopausal women; premenopausal women; progesterone; protein kinase B; randomized clinical trial; spontaneously hypertensive rat; testosterone; thromboxane A2; tumor necrosis factor-α; vascular smooth muscle; venous thrombo-embolism.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Vascular effects of estrogenic menopausal hormone therapy.Rev Recent Clin Trials. 2012 Feb;7(1):47-70. doi: 10.2174/157488712799363253. Rev Recent Clin Trials. 2012. PMID: 21864249 Free PMC article. Review.
-
Estrogenic compounds, estrogen receptors and vascular cell signaling in the aging blood vessels.Curr Med Chem. 2009;16(15):1863-87. doi: 10.2174/092986709788186093. Curr Med Chem. 2009. PMID: 19442151 Free PMC article. Review.
-
Modulators of vascular sex hormone receptors and their effects in estrogen-deficiency states associated with menopause.Recent Pat Cardiovasc Drug Discov. 2008 Nov;3(3):165-86. doi: 10.2174/157489008786263970. Recent Pat Cardiovasc Drug Discov. 2008. PMID: 18991792 Review.
-
Impact of sex hormone metabolism on the vascular effects of menopausal hormone therapy in cardiovascular disease.Curr Drug Metab. 2010 Oct;11(8):693-714. doi: 10.2174/138920010794233477. Curr Drug Metab. 2010. PMID: 21189141 Free PMC article. Review.
-
Research into Specific Modulators of Vascular Sex Hormone Receptors in the Management of Postmenopausal Cardiovascular Disease.Curr Hypertens Rev. 2009 Nov;5(4):283-306. doi: 10.2174/157340209789587717. Curr Hypertens Rev. 2009. PMID: 20694192 Free PMC article.
Cited by
-
Effects of Gender and Vitamin D on Vascular Reactivity of the Carotid Artery on a Testosterone-Induced PCOS Model.Int J Mol Sci. 2023 Nov 21;24(23):16577. doi: 10.3390/ijms242316577. Int J Mol Sci. 2023. PMID: 38068901 Free PMC article.
-
Examining the influence of wealth status on prehypertension risk in women aged 30-49: evidence from the 2018 Benin demographic and health survey.BMC Res Notes. 2024 Jan 2;17(1):10. doi: 10.1186/s13104-023-06676-6. BMC Res Notes. 2024. PMID: 38169420 Free PMC article.
-
Cardiac tissue remodeling in healthy aging: the road to pathology.Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C166-C182. doi: 10.1152/ajpcell.00021.2020. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432929 Free PMC article. Review.
-
Contraceptive Strategies in Women With Heart Failure or With Cardiac Transplantation.Curr Heart Fail Rep. 2018 Jun;15(3):161-170. doi: 10.1007/s11897-018-0392-x. Curr Heart Fail Rep. 2018. PMID: 29616492 Review.
-
The challenge of translating ischemic conditioning from animal models to humans: the role of comorbidities.Dis Model Mech. 2014 Dec;7(12):1321-33. doi: 10.1242/dmm.016741. Dis Model Mech. 2014. PMID: 25481012 Free PMC article. Review.
References
-
- Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease: what went wrong and where do we go from here? Hypertension. 2004;44:789–795. - PubMed
-
- Orshal JM, Khalil RA. Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2004;286:R233–R249. - PubMed
-
- Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118(Suppl 12B):64–73. - PubMed
-
- Rosano GM, Leonardo F, Pagnotta P, Pelliccia F, Panina G, Cerquetani E, et al. Acute anti-ischemic effect of testosterone in men with coronary artery disease. Circulation. 1999;99:1666–1670. - PubMed
-
- Eckstein N, Nadler E, Barnea O, Shavit G, Ayalon D. Acute effects of 17 beta-estradiol on the rat heart. Am J Obstet Gynecol. 1994;171:844–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

