A single point mutation reveals gating of the human ClC-5 Cl-/H+ antiporter
- PMID: 24099800
- PMCID: PMC3872759
- DOI: 10.1113/jphysiol.2013.260240
A single point mutation reveals gating of the human ClC-5 Cl-/H+ antiporter
Abstract
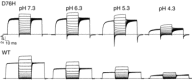
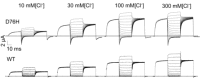
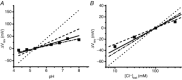
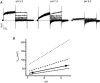
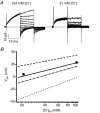
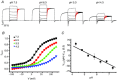
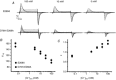
ClC-5 is a 2Cl(-)/1H(+) antiporter highly expressed in endosomes of proximal tubule cells. It is essential for endocytosis and mutations in ClC-5 cause Dent's disease, potentially leading to renal failure. However, the physiological role of ClC-5 is still unclear. One of the main issues is whether the strong rectification of ClC-5 currents observed in heterologous systems, with currents elicited only at positive voltages, is preserved in vivo and what is the origin of this rectification. In this work we identified a ClC-5 mutation, D76H, which, besides the typical outward currents of the wild-type (WT), shows inward tail currents at negative potentials that allow the estimation of the reversal of ClC-5 currents for the first time. A detailed analysis of the dependence of these inward tail currents on internal and external pH and [Cl(-)] shows that they are generated by a coupled transport of Cl(-) and H(+) with a 2 : 1 stoichiometry. From this result we conclude that the inward tail currents are caused by a gating mechanism that regulates ClC-5 transport activity and not by a major alteration of the transport mechanism itself. This implies that the strong rectification of the currents of WT ClC-5 is at least in part caused by a gating mechanism that activates the transporter at positive potentials. These results elucidate the biophysical properties of ClC-5 and contribute to the understanding of its physiological role.
Figures


References
-
- Accardi A, Miller C. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 2004;427:803–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

