Androgen induces a switch from cytoplasmic retention to nuclear import of the androgen receptor
- PMID: 24100013
- PMCID: PMC3889559
- DOI: 10.1128/MCB.00647-13
Androgen induces a switch from cytoplasmic retention to nuclear import of the androgen receptor
Abstract
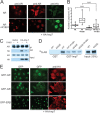
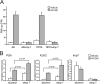
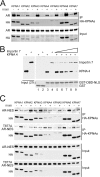
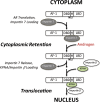
The androgen receptor (AR) has critical functions as a transcription factor in both normal and cancer cells, but the specific mechanisms that regulate its nuclear localization are not well defined. We found that an AR mutation commonly reported in prostate cancer generates an androgen-independent gain of function for nuclear import. The substitution, Thr877Ala, is within the ligand-binding domain, but the nuclear import gain of function is mediated by the bipartite nuclear localization signal (NLS) spanning the DNA-binding domain (DBD) and hinge region. Bipartite NLS activity depends on the structure provided by the DBD, and protein interactions with the bipartite NLS are repressed by the hinge region. The bipartite NLS is recognized by importin 7, a nuclear import receptor for several proteins. Importin 7 binding to AR, however, inhibits import by shielding the bipartite NLS. Androgen binding relieves the inhibition by inducing a switch that promotes exchange of importin 7 for karyopherin alpha import receptors. Importin 7 contributes to the regulation of AR import by restraining import until androgen is detected in the cytoplasm.
Figures





Similar articles
-
Characterization of nuclear import of the domain-specific androgen receptor in association with the importin alpha/beta and Ran-guanosine 5'-triphosphate systems.Endocrinology. 2008 Aug;149(8):3960-9. doi: 10.1210/en.2008-0137. Epub 2008 Apr 17. Endocrinology. 2008. PMID: 18420738 Free PMC article.
-
Structural basis for the nuclear import of the human androgen receptor.J Cell Sci. 2008 Apr 1;121(Pt 7):957-68. doi: 10.1242/jcs.022103. Epub 2008 Mar 4. J Cell Sci. 2008. PMID: 18319300
-
Androgen receptor splice variants activate androgen receptor target genes and support aberrant prostate cancer cell growth independent of canonical androgen receptor nuclear localization signal.J Biol Chem. 2012 Jun 1;287(23):19736-49. doi: 10.1074/jbc.M112.352930. Epub 2012 Apr 24. J Biol Chem. 2012. PMID: 22532567 Free PMC article.
-
The importin β binding domain as a master regulator of nucleocytoplasmic transport.Biochim Biophys Acta. 2011 Sep;1813(9):1578-92. doi: 10.1016/j.bbamcr.2010.10.012. Epub 2010 Oct 26. Biochim Biophys Acta. 2011. PMID: 21029753 Free PMC article. Review.
-
Bipartite nuclear localization sequence is indispensable for nuclear import and stability of self-dimerization of ADARa in Bombyx mori.Insect Biochem Mol Biol. 2024 Nov;174:104190. doi: 10.1016/j.ibmb.2024.104190. Epub 2024 Oct 9. Insect Biochem Mol Biol. 2024. PMID: 39389319 Review.
Cited by
-
Targeting of CYP17A1 Lyase by VT-464 Inhibits Adrenal and Intratumoral Androgen Biosynthesis and Tumor Growth of Castration Resistant Prostate Cancer.Sci Rep. 2016 Oct 17;6:35354. doi: 10.1038/srep35354. Sci Rep. 2016. PMID: 27748439 Free PMC article.
-
Acetylation of androgen receptor by ARD1 promotes dissociation from HSP90 complex and prostate tumorigenesis.Oncotarget. 2016 Nov 1;7(44):71417-71428. doi: 10.18632/oncotarget.12163. Oncotarget. 2016. PMID: 27659526 Free PMC article.
-
Polyglutamine androgen receptor-mediated neuromuscular disease.Cell Mol Life Sci. 2016 Nov;73(21):3991-9. doi: 10.1007/s00018-016-2275-1. Epub 2016 May 17. Cell Mol Life Sci. 2016. PMID: 27188284 Free PMC article. Review.
-
Androgen Receptor Dependence.Adv Exp Med Biol. 2019;1210:333-350. doi: 10.1007/978-3-030-32656-2_15. Adv Exp Med Biol. 2019. PMID: 31900916 Free PMC article. Review.
-
Molecular Mechanisms and Therapeutics for SBMA/Kennedy's Disease.Neurotherapeutics. 2019 Oct;16(4):928-947. doi: 10.1007/s13311-019-00790-9. Neurotherapeutics. 2019. PMID: 31686397 Free PMC article. Review.
References
-
- Pemberton LF, Paschal BM. 2005. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic 6:187–198 - PubMed
-
- Harel A, Forbes DJ. 2004. Importin beta: conducting a much larger cellular symphony. Mol. Cell 16:319–330 - PubMed
-
- Goldfarb DS, Corbett AH, Mason DA, Harreman MT, Adam SA. 2004. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 14:505–514 - PubMed
-
- Gorlich D, Vogel F, Mills AD, Hartmann E, Laskey RA. 1995. Distinct functions for the two importin subunits in nuclear protein import. Nature 377:246–248 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
