Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset
- PMID: 24100295
- PMCID: PMC3798251
- DOI: 10.1083/jcb.201302060
Sequestration of CDH1 by MAD2L2 prevents premature APC/C activation prior to anaphase onset
Abstract
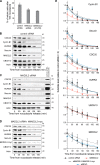
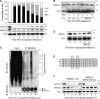
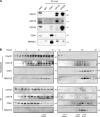
The switch from activation of the anaphase-promoting complex/cyclosome (APC/C) by CDC20 to CDH1 during anaphase is crucial for accurate mitosis. APC/C(CDC20) ubiquitinates a limited set of substrates for subsequent degradation, including Cyclin B1 and Securin, whereas APC/C(CDH1) has a broader specificity. This switch depends on dephosphorylation of CDH1 and the APC/C, and on the degradation of CDC20. Here we show, in human cells, that the APC/C inhibitor MAD2L2 also contributes to ensuring the sequential activation of the APC/C by CDC20 and CDH1. In prometaphase, MAD2L2 sequestered free CDH1 away from the APC/C. At the onset of anaphase, MAD2L2 was rapidly degraded by APC/C(CDC20), releasing CDH1 to activate the dephosphorylated APC/C. Loss of MAD2L2 led to premature association of CDH1 with the APC/C, early destruction of APC/C(CDH1) substrates, and accelerated mitosis with frequent mitotic aberrations. Thus, MAD2L2 helps to ensure a robustly bistable switch between APC/C(CDC20) and APC/C(CDH1) during the metaphase-to-anaphase transition, thereby contributing to mitotic fidelity.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

