Approaches to the synthesis of a novel, anti-HIV active integrase inhibitor
- PMID: 24100441
- PMCID: PMC3846259
- DOI: 10.1039/c3ob41728j
Approaches to the synthesis of a novel, anti-HIV active integrase inhibitor
Abstract
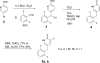
The novel HIV-1 integrase inhibitor 1, discovered in our laboratory, exhibits potent anti-HIV activity against a diverse set of HIV-1 isolates and also against HIV-2 and SIV. In addition, this compound displays low cellular cytotoxicity and possesses a favorable in vitro drug interaction profile with respect to isozymes of cytochrome P450 (CYP) and uridine 5'-diphospho-glucuronosyltransferase (UGT). However, the total synthesis of this significant HIV integrase inhibitor has not been reported. This contribution describes an optimized, reproducible, multi-step, synthetic route to inhibitor 1. The yield for the separate steps averaged about 80%. The methodologies utilized in the synthesis were, among others, a palladium-catalyzed cross-coupling reaction, a crossed-Claisen condensation, and a hydrazino amide synthesis step. Successful alternative synthetic methodologies for some of the steps are also described.
Figures


Similar articles
-
A novel anti-HIV active integrase inhibitor with a favorable in vitro cytochrome P450 and uridine 5'-diphospho-glucuronosyltransferase metabolism profile.Antiviral Res. 2013 Jun;98(3):365-72. doi: 10.1016/j.antiviral.2013.04.005. Epub 2013 Apr 18. Antiviral Res. 2013. PMID: 23602851 Free PMC article.
-
Notable difference in anti-HIV activity of integrase inhibitors as a consequence of geometric and enantiomeric configurations.Bioorg Med Chem Lett. 2013 Jul 15;23(14):4112-6. doi: 10.1016/j.bmcl.2013.05.050. Epub 2013 May 23. Bioorg Med Chem Lett. 2013. PMID: 23746474 Free PMC article.
-
Design, synthesis and SAR study of novel C2-pyrazolopyrimidine amides and amide isosteres as allosteric integrase inhibitors.Bioorg Med Chem Lett. 2020 Nov 1;30(21):127516. doi: 10.1016/j.bmcl.2020.127516. Epub 2020 Aug 27. Bioorg Med Chem Lett. 2020. PMID: 32860982
-
Integrase Inhibitor Prodrugs: Approaches to Enhancing the Anti-HIV Activity of β-Diketo Acids.Molecules. 2015 Jul 13;20(7):12623-51. doi: 10.3390/molecules200712623. Molecules. 2015. PMID: 26184144 Free PMC article. Review.
-
Novel inhibitors of HIV integrase: the discovery of potential anti-HIV therapeutic agents.Curr Pharm Des. 2003;9(31):2553-65. doi: 10.2174/1381612033453703. Curr Pharm Des. 2003. PMID: 14529542 Review.
Cited by
-
A novel molecule with notable activity against multi-drug resistant tuberculosis.Bioorg Med Chem Lett. 2015 Mar 15;25(6):1269-73. doi: 10.1016/j.bmcl.2015.01.050. Epub 2015 Jan 28. Bioorg Med Chem Lett. 2015. PMID: 25677656 Free PMC article.
-
Recent Developments in the Synthesis of HIV-1 Integrase Strand Transfer Inhibitors Incorporating Pyridine Moiety.Int J Mol Sci. 2023 May 26;24(11):9314. doi: 10.3390/ijms24119314. Int J Mol Sci. 2023. PMID: 37298265 Free PMC article. Review.
-
Pharmacokinetics and dose-range finding toxicity of a novel anti-HIV active integrase inhibitor.Antiviral Res. 2014 Aug;108:25-9. doi: 10.1016/j.antiviral.2014.05.001. Epub 2014 May 10. Antiviral Res. 2014. PMID: 24821255 Free PMC article.
References
-
- Dye C, Williams BG. Science. 2010;328:856–861. - PubMed
-
- Trono D, Van Lint C, Rouzioux C, Verdin E, Barre-Sinoussi F, Chun TW, Chomont N. Science. 2010;329:174–180. - PubMed
-
- Josephson F. J. Intern. Med. 2010;268:530–539. - PubMed
-
- Kiang TKL, Ensom MHH, Chang TKH. Pharmacol. Therapeut. 2005;106:97–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

