Megalin contributes to kidney accumulation and nephrotoxicity of colistin
- PMID: 24100504
- PMCID: PMC3837877
- DOI: 10.1128/AAC.00254-13
Megalin contributes to kidney accumulation and nephrotoxicity of colistin
Abstract
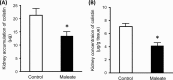
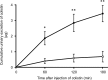
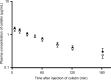
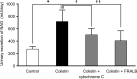
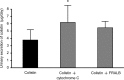
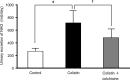
Interest has recently been shown again in colistin because of the increased prevalence of infections caused by multidrug-resistant Gram-negative bacteria. Although the potential for nephrotoxicity is a major dose-limiting factor in colistin use, little is known about the mechanisms that underlie colistin-induced nephrotoxicity. In this study, we focused on an endocytosis receptor, megalin, that is expressed in renal proximal tubules, with the aim of clarifying the role of megalin in the kidney accumulation and nephrotoxicity of colistin. We examined the binding of colistin to megalin by using a vesicle assay. The kidney accumulation, urinary excretion, and concentrations in plasma of colistin in megalin-shedding rats were also evaluated. Furthermore, we examined the effect of megalin ligands and a microtubule-depolymerizing agent on colistin-induced nephrotoxicity. We found that cytochrome c, a typical megalin ligand, inhibited the binding of colistin to megalin competitively. In megalin-shedding rats, renal proximal tubule colistin accumulation was decreased (13.5 ± 1.6 and 21.3 ± 2.6 μg in megalin-shedding and control rats, respectively). Coadministration of colistin and cytochrome c or albumin fragments resulted in a significant decrease in urinary N-acetyl-β-d-glucosaminidase (NAG) excretion, a marker of renal tubular damage (717.1 ± 183.9 mU/day for colistin alone, 500.8 ± 102.4 mU/day for cytochrome c with colistin, and 406.7 ± 156.7 mU/day for albumin fragments with colistin). Moreover, coadministration of colistin and colchicine, a microtubule-depolymerizing agent, resulted in a significant decrease in urinary NAG excretion. In conclusion, our results indicate that colistin acts as a megalin ligand and that megalin plays a key role in the accumulation in the kidney and nephrotoxicity of colistin. Megalin ligands may be new targets for the prevention of colistin-induced nephrotoxicity.
Figures


Similar articles
-
Megalin Blockade with Cilastatin Suppresses Drug-Induced Nephrotoxicity.J Am Soc Nephrol. 2017 Jun;28(6):1783-1791. doi: 10.1681/ASN.2016060606. Epub 2017 Jan 4. J Am Soc Nephrol. 2017. PMID: 28052987 Free PMC article.
-
Preliminary clinical study of the effect of ascorbic acid on colistin-associated nephrotoxicity.Antimicrob Agents Chemother. 2015;59(6):3224-32. doi: 10.1128/AAC.00280-15. Epub 2015 Mar 23. Antimicrob Agents Chemother. 2015. PMID: 25801556 Free PMC article. Clinical Trial.
-
Targeted prevention of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists.J Control Release. 2004 Mar 24;95(3):423-33. doi: 10.1016/j.jconrel.2003.12.005. J Control Release. 2004. PMID: 15023454
-
The endocytosis receptor megalin: From bench to bedside.Int J Biochem Cell Biol. 2023 Apr;157:106393. doi: 10.1016/j.biocel.2023.106393. Epub 2023 Feb 28. Int J Biochem Cell Biol. 2023. PMID: 36863658 Review.
-
Molecular Mechanisms of Colistin-Induced Nephrotoxicity.Molecules. 2019 Feb 12;24(3):653. doi: 10.3390/molecules24030653. Molecules. 2019. PMID: 30759858 Free PMC article. Review.
Cited by
-
Intracellular localization of polymyxins in human alveolar epithelial cells.J Antimicrob Chemother. 2019 Jan 1;74(1):48-57. doi: 10.1093/jac/dky409. J Antimicrob Chemother. 2019. PMID: 30357331 Free PMC article.
-
Pharmacological agents for the prevention of colistin-induced nephrotoxicity.Eur J Med Res. 2022 May 7;27(1):64. doi: 10.1186/s40001-022-00689-w. Eur J Med Res. 2022. PMID: 35525994 Free PMC article. Review.
-
Reply to "measuring polymyxin uptake by renal tubular cells: is BODIPY-polymyxin B an appropriate probe?".Antimicrob Agents Chemother. 2014 Oct;58(10):6339. doi: 10.1128/AAC.03784-14. Antimicrob Agents Chemother. 2014. PMID: 25225341 Free PMC article. No abstract available.
-
Synchrotron-based X-ray fluorescence microscopy reveals accumulation of polymyxins in single human alveolar epithelial cells.Antimicrob Agents Chemother. 2023 May 1;65(5):e02314-20. doi: 10.1128/AAC.02314-20. Epub 2021 Mar 1. Antimicrob Agents Chemother. 2023. PMID: 33649114 Free PMC article.
-
[Does dexmedetomidine prevent colistin nephrotoxicity?].Braz J Anesthesiol. 2018 Jul-Aug;68(4):383-387. doi: 10.1016/j.bjan.2018.01.017. Epub 2018 Jun 7. Braz J Anesthesiol. 2018. PMID: 29885938 Free PMC article.
References
-
- Gudiol C, Tubau F, Calatayud L, Garcia-Vidal C, Cisnal M, Sánchez-Ortega I, Duarte R, Calvo M, Carratalà J. 2011. Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J. Antimicrob. Chemother. 66:657–663 - PubMed
-
- Leibovici L, Samra Z, Konigsberger H, Drucker M, Ashkenazi S, Pitlik SD. 1995. Long-term survival following bacteremia or fungemia. JAMA 274:807–812 - PubMed
-
- Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, Giasnetsova T, Tsioka A, Roilides E, Sofianou D, Gritsi-Gerogianni N. 2010. Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing K. pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect. Control Hosp. Epidemiol. 31:1250–1256 - PubMed
-
- Boyd N, Nailor MD. 2011. Combination antibiotic therapy for empiric and definitive treatment of Gram-negative infections: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 31:1073–1084 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

