Novel tools to dissect the dynamic regulation of TCR signaling by the kinase Csk and the phosphatase CD45
- PMID: 24100586
- PMCID: PMC4039614
- DOI: 10.1101/sqb.2013.78.020347
Novel tools to dissect the dynamic regulation of TCR signaling by the kinase Csk and the phosphatase CD45
Abstract
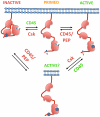
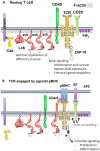
Although the biochemical events induced by T-cell receptor (TCR) triggering have been well studied, both the mediators and function of basal signaling in T cells remain poorly understood. Furthermore, the precise mechanisms by which MHC-peptide interaction with the TCR disrupt the basal equilibrium to induce downstream signaling are also unclear. Here we describe novel approaches to understand the basal state of T cells and the mechanisms of TCR triggering by perturbing regulation of the Src family kinases (SFKs). The SFKs are critical proximal mediators of TCR signaling that are in turn tightly regulated by the tyrosine kinase Csk and the receptor-like tyrosine phosphatase CD45. We have developed a small-molecule analog-sensitive allele of Csk and an allelic series of mice in which expression of CD45 is varied across a broad range. Our studies have unmasked contributions of Csk and CD45 to maintain the basal state of T cells and also suggest that dynamic regulation of Csk may be involved in TCR triggering.
Copyright © 2013 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


Similar articles
-
CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development.Immunity. 2010 Mar 26;32(3):342-54. doi: 10.1016/j.immuni.2010.03.006. Immunity. 2010. PMID: 20346773 Free PMC article.
-
Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling.Sci Signal. 2011 Sep 13;4(190):ra59. doi: 10.1126/scisignal.2001893. Sci Signal. 2011. PMID: 21917715 Free PMC article.
-
Regulation of Src family kinases involved in T cell receptor signaling by protein-tyrosine phosphatase CD148.J Biol Chem. 2011 Jun 24;286(25):22101-12. doi: 10.1074/jbc.M110.196733. Epub 2011 May 4. J Biol Chem. 2011. PMID: 21543337 Free PMC article.
-
Negative regulation of antigen receptor signaling in lymphocytes.J Mol Med (Berl). 1998 Jul;76(8):589-95. doi: 10.1007/s001090050254. J Mol Med (Berl). 1998. PMID: 9694436 Review.
-
The regulation of antigen-receptor signaling by protein tyrosine phosphatases: a hole in the story.Curr Opin Immunol. 1999 Jun;11(3):270-6. doi: 10.1016/s0952-7915(99)80044-2. Curr Opin Immunol. 1999. PMID: 10375547 Review.
Cited by
-
The role of T cell receptor signaling thresholds in guiding T cell fate decisions.Curr Opin Immunol. 2015 Apr;33:43-8. doi: 10.1016/j.coi.2015.01.012. Epub 2015 Feb 6. Curr Opin Immunol. 2015. PMID: 25660212 Free PMC article. Review.
-
Initiation of T cell signaling by CD45 segregation at 'close contacts'.Nat Immunol. 2016 May;17(5):574-582. doi: 10.1038/ni.3392. Epub 2016 Mar 21. Nat Immunol. 2016. PMID: 26998761 Free PMC article.
-
Programmable DNA-augmented hydrogels for controlled activation of human lymphocytes.Nanomedicine. 2021 Oct;37:102442. doi: 10.1016/j.nano.2021.102442. Epub 2021 Jul 17. Nanomedicine. 2021. PMID: 34284132 Free PMC article.
-
In vitro membrane reconstitution of the T-cell receptor proximal signaling network.Nat Struct Mol Biol. 2014 Feb;21(2):133-42. doi: 10.1038/nsmb.2762. Epub 2014 Jan 26. Nat Struct Mol Biol. 2014. PMID: 24463463 Free PMC article.
-
The catalytic activity of the kinase ZAP-70 mediates basal signaling and negative feedback of the T cell receptor pathway.Sci Signal. 2015 May 19;8(377):ra49. doi: 10.1126/scisignal.2005596. Sci Signal. 2015. PMID: 25990959 Free PMC article.
References
-
- Ashwell JD, D'Oro U. CD45 and Src-family kinases: and now for something completely different. Immunol Today. 1999;20(9):412–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
