Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment
- PMID: 24100640
- PMCID: PMC3944332
- DOI: 10.1038/mt.2013.237
Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment
Abstract
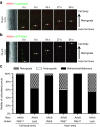
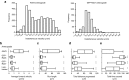
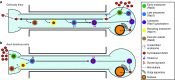
Adeno-associated virus (AAV) vectors can move along axonal pathways after brain injection, resulting in transduction of distal brain regions. This can enhance the spread of therapeutic gene transfer and improve treatment of neurogenetic disorders that require global correction. To better understand the underlying cellular mechanisms that drive AAV trafficking in neurons, we investigated the axonal transport of dye-conjugated AAV9, utilizing microfluidic primary neuron cultures that isolate cell bodies from axon termini and permit independent analysis of retrograde and anterograde axonal transport. After entry, AAV was trafficked into nonmotile early and recycling endosomes, exocytic vesicles, and a retrograde-directed late endosome/lysosome compartment. Rab7-positive late endosomes/lysosomes that contained AAV were highly motile, exhibiting faster retrograde velocities and less pausing than Rab7-positive endosomes without virus. Inhibitor experiments indicated that the retrograde transport of AAV within these endosomes is driven by cytoplasmic dynein and requires Rab7 function, whereas anterograde transport of AAV is driven by kinesin-2 and exhibits unusually rapid velocities. Furthermore, increasing AAV9 uptake by neuraminidase treatment significantly enhanced virus transport in both directions. These findings provide novel insights into AAV trafficking within neurons, which should enhance progress toward the utilization of AAV for improved distribution of transgene delivery within the brain.
Figures





References
-
- Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. - PubMed
-
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat Protoc. 2006;1:1412–1428. - PubMed
-
- Rivera VM, Gao GP, Grant RL, Schnell MA, Zoltick PW, Rozamus LW, et al. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood. 2005;105:1424–1430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

