Efficacy of the dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with nilotinib against BCR-ABL-positive leukemia cells involves the ABL kinase domain mutation
- PMID: 24100660
- PMCID: PMC3928137
- DOI: 10.4161/cbt.26725
Efficacy of the dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with nilotinib against BCR-ABL-positive leukemia cells involves the ABL kinase domain mutation
Abstract
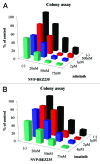
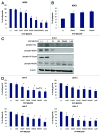
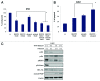
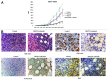
Imatinib, an ABL tyrosine kinase inhibitor (TKI), has shown clinical efficacy against chronic myeloid leukemia (CML). However, a substantial number of patients develop resistance to imatinib treatment due to the emergence of clones carrying mutations in the protein BCR-ABL. The phosphoinositide 3 kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway regulates various processes, including cell proliferation, cell survival, and antiapoptosis activity. In this study, we investigated the efficacy of NVP-BEZ235, a dual PI3K and mTOR inhibitor, using BCR-ABL-positive cell lines. Treatment with NVP-BEZ235 for 48 h inhibited cell growth and induced apoptosis. The phosphorylation of the AKT kinase, eukaryotic initiation factor 4-binding protein 1 (4E-BP1), and p70 S6 kinase were decreased after NVP-BEZ235 treatment. The combination of NVP-BEZ235 with a BCR-ABL kinase inhibitor, imatinib, or nilotinib, induced a more pronounced colony growth inhibition, whereas the combination of NVP-BEZ235 and nilotinib was more effective in inducing apoptosis and reducing the phosphorylation of AKT, 4E-BP1, and S6 kinase. NVP-BEZ235 in combination with nilotinib also inhibited tumor growth in a xenograft model and inhibited the growth of primary T315I mutant cells and ponatinib-resistant cells. Taken together, these results suggest that administration of the dual PI3K and mTOR inhibitor NVP-BEZ235 may be an effective strategy against BCR-ABL mutant cells and may enhance the cytotoxic effects of nilotinib in ABL TKI-resistant BCR-ABL mutant cells.
Keywords: BCR-ABL mutation; NVP-BEZ235; PI3K; chronic myeloid leukemia; mTOR; nilotinib; ponatinib resistance.
Figures
Similar articles
-
Targeting BCR-ABL-Independent TKI Resistance in Chronic Myeloid Leukemia by mTOR and Autophagy Inhibition.J Natl Cancer Inst. 2018 May 1;110(5):467-478. doi: 10.1093/jnci/djx236. J Natl Cancer Inst. 2018. PMID: 29165716 Free PMC article.
-
Efficacy of the dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with imatinib mesylate against chronic myelogenous leukemia cell lines.Drug Des Devel Ther. 2017 Apr 3;11:1115-1126. doi: 10.2147/DDDT.S132092. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28435223 Free PMC article.
-
Gab2 signaling in chronic myeloid leukemia cells confers resistance to multiple Bcr-Abl inhibitors.Leukemia. 2013 Jan;27(1):118-29. doi: 10.1038/leu.2012.222. Epub 2012 Aug 3. Leukemia. 2013. PMID: 22858987
-
Combating TKI resistance in CML by inhibiting the PI3K/Akt/mTOR pathway in combination with TKIs: a review.Med Oncol. 2021 Jan 16;38(1):10. doi: 10.1007/s12032-021-01462-5. Med Oncol. 2021. PMID: 33452624 Review.
-
New Bcr-Abl inhibitors in chronic myeloid leukemia: keeping resistance in check.Expert Opin Investig Drugs. 2008 Jun;17(6):865-78. doi: 10.1517/13543784.17.6.865. Expert Opin Investig Drugs. 2008. PMID: 18491988 Review.
Cited by
-
Therapeutic potential of targeting sphingosine kinases and sphingosine 1-phosphate in hematological malignancies.Leukemia. 2016 Nov;30(11):2142-2151. doi: 10.1038/leu.2016.208. Epub 2016 Jul 27. Leukemia. 2016. PMID: 27461062 Review.
-
Selective anti-cancer agents as anti-aging drugs.Cancer Biol Ther. 2013 Dec;14(12):1092-7. doi: 10.4161/cbt.27350. Epub 2013 Nov 27. Cancer Biol Ther. 2013. PMID: 24345884 Free PMC article.
-
Differentiation status of primary chronic myeloid leukemia cells affects sensitivity to BCR-ABL1 inhibitors.Oncotarget. 2017 Apr 4;8(14):22606-22615. doi: 10.18632/oncotarget.15146. Oncotarget. 2017. PMID: 28186983 Free PMC article.
-
Investigation of TSRP reverses imatinib resistance through the PI3K / Akt pathway in chronic myeloid leukemia.Ann Hematol. 2024 Dec;103(12):5285-5296. doi: 10.1007/s00277-024-06099-8. Epub 2024 Nov 25. Ann Hematol. 2024. PMID: 39586883
-
Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells.Cancer Biol Ther. 2015;16(4):567-79. doi: 10.1080/15384047.2015.1018494. Epub 2015 Apr 11. Cancer Biol Ther. 2015. PMID: 25866016 Free PMC article.
References
-
- Nowell PC, Hungerford DA. A minute chromosome in human granulocytic leukemia. Science. 1960;142:1497.
-
- Tauchi T, Okabe S, Miyazawa K, Ohyashiki K. The tetramerization domain-independent Ras activation by BCR-ABL oncoprotein in hematopoietic cells. Int J Oncol. 1998;12:1269–76. - PubMed
-
- Skorski T, Kanakaraj P, Nieborowska-Skorska M, Ratajczak MZ, Wen SC, Zon G, Gewirtz AM, Perussia B, Calabretta B. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86:726–36. - PubMed
-
- Chai SK, Nichols GL, Rothman P. Constitutive activation of JAKs and STATs in BCR-Abl-expressing cell lines and peripheral blood cells derived from leukemic patients. J Immunol. 1997;159:4720–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
