Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome
- PMID: 24101038
- PMCID: PMC5569679
- DOI: 10.1200/JCO.2013.50.4910
Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome
Abstract
Purpose: A multicenter phase II study was conducted to assess the efficacy of rituximab, methotrexate, procarbazine, and vincristine (R-MPV) followed by consolidation reduced-dose whole-brain radiotherapy (rdWBRT) and cytarabine in primary CNS lymphoma.
Patients and methods: Patients received induction chemotherapy with R-MPV (five to seven cycles); those achieving a complete response (CR) received rdWBRT (23.4 Gy), and otherwise, standard WBRT was offered (45 Gy). Consolidation cytarabine was given after the radiotherapy. The primary end point was 2-year progression-free survival (PFS) in patients receiving rdWBRT. Exploratory end points included prospective neuropsychological evaluation, analysis of magnetic resonance imaging (MRI) white matter changes using the Fazekas scale, and evaluation of the apparent diffusion coefficient (ADC) as a prognostic factor.
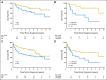
Results: Fifty-two patients were enrolled, with median age of 60 years (range, 30 to 79 years) and median Karnofsky performance score of 70 (range, 50 to 100). Thirty-one patients (60%) achieved a CR after R-MPV and received rdWBRT. The 2-year PFS for this group was 77%; median PFS was 7.7 years. Median overall survival (OS) was not reached (median follow-up for survivors, 5.9 years); 3-year OS was 87%. The overall (N = 52) median PFS was 3.3 years, and median OS was 6.6 years. Cognitive assessment showed improvement in executive function (P < .01) and verbal memory (P < .05) after chemotherapy, and follow-up scores remained relatively stable across the various domains (n = 12). All examined MRIs (n = 28) displayed a Fazekas score of ≤ 3, and no patient developed scores of 4 to 5; differences in ADC values did not predict response (P = .15), PFS (P = .27), or OS (P = .33).
Conclusion: R-MPV combined with consolidation rdWBRT and cytarabine is associated with high response rates, long-term disease control, and minimal neurotoxicity.
Trial registration: ClinicalTrials.gov NCT00594815.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Morris PG, Abrey LE: Therapeutic challenges in primary CNS lymphoma Lancet Neurol 8:581–592,2009 - PubMed
-
- Ferreri AJ DeAngelis L Illerhaus G, etal: Whole-brain radiotherapy in primary CNS lymphoma Lancet Oncol 12:118–119,2011 - PubMed
-
- Korfel A Thiel E Martus P, etal: Whole-brain radiotherapy in primary CNS lymphoma (authors' reply) Lancet Oncol 12:119–120,2011 - PubMed
-
- Graber JJ, Omuro A: Primary central nervous system lymphoma: Is there still a role for radiotherapy? Curr Opin Neurol 24:633–640,2011 - PubMed
-
- Thiel E Korfel A Martus P, etal: High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial Lancet Oncol 11:1036–1047,2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

