Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial
- PMID: 24101040
- PMCID: PMC3816958
- DOI: 10.1200/JCO.2013.49.6968
Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial
Abstract
Purpose: Radiotherapy with concomitant and adjuvant temozolomide is the standard of care for newly diagnosed glioblastoma (GBM). O(6)-methylguanine-DNA methyltransferase (MGMT) methylation status may be an important determinant of treatment response. Dose-dense (DD) temozolomide results in prolonged depletion of MGMT in blood mononuclear cells and possibly in tumor. This trial tested whether DD temozolomide improves overall survival (OS) or progression-free survival (PFS) in patients with newly diagnosed GBM.
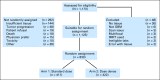
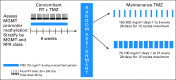
Patients and methods: This phase III trial enrolled patients older than age 18 years with a Karnofsky performance score of ≥ 60 with adequate tissue. Stratification included clinical factors and tumor MGMT methylation status. Patients were randomly assigned to standard temozolomide (arm 1) or DD temozolomide (arm 2) for 6 to 12 cycles. The primary end point was OS. Secondary analyses evaluated the impact of MGMT status.
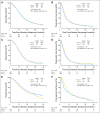
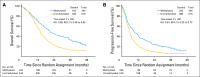
Results: A total of 833 patients were randomly assigned to either arm 1 or arm 2 (1,173 registered). No statistically significant difference was observed between arms for median OS (16.6 v 14.9 months, respectively; hazard ratio [HR], 1.03; P = .63) or median PFS (5.5 v 6.7 months; HR, 0.87; P = .06). Efficacy did not differ by methylation status. MGMT methylation was associated with improved OS (21.2 v 14 months; HR, 1.74; P < .001), PFS (8.7 v 5.7 months; HR, 1.63; P < .001), and response (P = .012). There was increased grade ≥ 3 toxicity in arm 2 (34% v 53%; P < .001), mostly lymphopenia and fatigue.
Conclusion: This study did not demonstrate improved efficacy for DD temozolomide for newly diagnosed GBM, regardless of methylation status. However, it did confirm the prognostic significance of MGMT methylation. Feasibility of large-scale accrual, prospective tumor collection, and molecular stratification was demonstrated.
Trial registration: ClinicalTrials.gov NCT00304031.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Lessons learned from Radiation Therapy Oncology Group 0525 trial.J Clin Oncol. 2014 May 20;32(15):1633-4. doi: 10.1200/JCO.2013.54.6226. Epub 2014 Apr 21. J Clin Oncol. 2014. PMID: 24752051 No abstract available.
-
Reply to M.C. Chamberlain.J Clin Oncol. 2014 May 20;32(15):1634-5. doi: 10.1200/JCO.2013.54.9717. Epub 2014 Apr 21. J Clin Oncol. 2014. PMID: 24752060 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. - PubMed
-
- Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. - PubMed
-
- Liu L, Gerson SL. Targeted modulation of MGMT: Clinical implications. Clin Cancer Res. 2006;12:328–331. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

