Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer
- PMID: 24101053
- PMCID: PMC4878106
- DOI: 10.1200/JCO.2012.47.4189
Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer
Abstract
Purpose: Increased hepatocyte growth factor/MET signaling is associated with poor prognosis and acquired resistance to epidermal growth factor receptor (EGFR) -targeted drugs in patients with non-small-cell lung cancer (NSCLC). We investigated whether dual inhibition of MET/EGFR results in clinical benefit in patients with NSCLC.
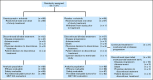
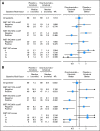
Patients and methods: Patients with recurrent NSCLC were randomly assigned at a ratio of one to one to receive onartuzumab plus erlotinib or placebo plus erlotinib; crossover was allowed at progression. Tumor tissue was required to assess MET status by immunohistochemistry (IHC). Coprimary end points were progression-free survival (PFS) in the intent-to-treat (ITT) and MET-positive (MET IHC diagnostic positive) populations; additional end points included overall survival (OS), objective response rate, and safety.
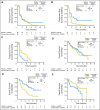
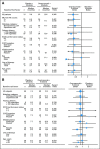
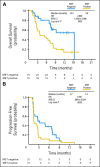
Results: There was no improvement in PFS or OS in the ITT population (n = 137; PFS hazard ratio [HR], 1.09; P = .69; OS HR, 0.80; P = .34). MET-positive patients (n = 66) treated with erlotinib plus onartuzumab showed improvement in both PFS (HR, .53; P = .04) and OS (HR, .37; P = .002). Conversely, clinical outcomes were worse in MET-negative patients treated with onartuzumab plus erlotinib (n = 62; PFS HR, 1.82; P = .05; OS HR, 1.78; P = .16). MET-positive control patients had worse outcomes versus MET-negative control patients (n = 62; PFS HR, 1.71; P = .06; OS HR, 2.61; P = .004). Incidence of peripheral edema was increased in onartuzumab-treated patients.
Conclusion: Onartuzumab plus erlotinib was associated with improved PFS and OS in the MET-positive population. These results combined with the worse outcomes observed in MET-negative patients treated with onartuzumab highlight the importance of diagnostic testing in drug development.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Signaling control by epidermal growth factor receptor and MET: rationale for cotargeting strategies in lung cancer.J Clin Oncol. 2013 Nov 10;31(32):4148-50. doi: 10.1200/JCO.2013.50.8234. Epub 2013 Oct 7. J Clin Oncol. 2013. PMID: 24101046 No abstract available.
-
Lung cancer: MET-negative patients--eclipsing benefits.Nat Rev Clin Oncol. 2013 Dec;10(12):667. doi: 10.1038/nrclinonc.2013.194. Epub 2013 Oct 22. Nat Rev Clin Oncol. 2013. PMID: 24145986 No abstract available.
-
Effect of onartuzumab added to erlotinib on metastasis in patients with lung cancer.J Clin Oncol. 2014 Nov 20;32(33):3781. doi: 10.1200/JCO.2013.54.3413. Epub 2014 Oct 20. J Clin Oncol. 2014. PMID: 25332242 No abstract available.
-
Reply to A. Soultati et Al.J Clin Oncol. 2014 Nov 20;32(33):3781-2. doi: 10.1200/JCO.2013.54.9683. Epub 2014 Oct 20. J Clin Oncol. 2014. PMID: 25332251 No abstract available.
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Shepherd FA. Current paradigms in first-line treatment of non-small-cell lung cancer. Oncology (Williston Park) 2004;18(suppl 5):13–20. - PubMed
-
- Patel JD. Epidermal growth factor receptor pathway targeted therapy in patients with aerodigestive malignancies. Curr Opin Oncol. 2006;18:609–614. - PubMed
-
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. - PubMed
-
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

