Expression and purification of recombinant human serum albumin from selectively terminable transgenic rice
- PMID: 24101203
- PMCID: PMC3796638
- DOI: 10.1631/jzus.B1300090
Expression and purification of recombinant human serum albumin from selectively terminable transgenic rice
Abstract
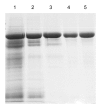
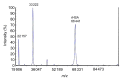
Human serum albumin (HSA) is widely utilized for medical purposes and biochemical research. Transgenic rice has proved to be an attractive bioreactor for mass production of recombinant HSA (rHSA). However, transgene spread is a major environmental and food safety concern for transgenic rice expressing proteins of medical value. This study aimed to develop a selectively terminable transgenic rice line expressing HSA in rice seeds, and a simple process for recovery and purification of rHSA for economical manufacture. An HSA expression cassette was inserted into a T-DNA vector encoding an RNA interference (RNAi) cassette suppressing the CYP81A6 gene. This gene detoxifies the herbicide bentazon and is linked to the 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) cassette which confers glyphosate tolerance. ANX Sepharose Fast Flow (ANX FF) anion exchange chromatography coupled with Butyl Sepharose High Performance (Butyl HP) hydrophobic interaction chromatography was used to purify rHSA. A transgenic rice line, HSA-84, was obtained with stable expression of rHSA of up to 0.72% of the total dry weight of the dehusked rice seeds. This line also demonstrated high sensitivity to bentazon, and thus could be killed selectively by a spray of bentazon. A two-step chromatography purification scheme was established to purify the rHSA from rice seeds to a purity of 99% with a recovery of 62.4%. Results from mass spectrometry and N-terminus sequencing suggested that the purified rHSA was identical to natural plasma-derived HSA. This study provides an alternative strategy for large-scale production of HSA with a built-in transgene safety control mechanism.
Conflict of interest statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures





Similar articles
-
A double built-in containment strategy for production of recombinant proteins in transgenic rice.PLoS One. 2014 Dec 22;9(12):e115459. doi: 10.1371/journal.pone.0115459. eCollection 2014. PLoS One. 2014. PMID: 25531447 Free PMC article.
-
A selectively terminable transgenic rice line expressing human lactoferrin.Protein Expr Purif. 2010 Nov;74(1):60-4. doi: 10.1016/j.pep.2010.04.015. Epub 2010 Apr 28. Protein Expr Purif. 2010. PMID: 20433928
-
A built-in strategy for containment of transgenic plants: creation of selectively terminable transgenic rice.PLoS One. 2008 Mar 19;3(3):e1818. doi: 10.1371/journal.pone.0001818. PLoS One. 2008. PMID: 18350155 Free PMC article.
-
Human serum albumin from recombinant DNA technology: challenges and strategies.Biochim Biophys Acta. 2013 Dec;1830(12):5515-25. doi: 10.1016/j.bbagen.2013.04.037. Epub 2013 May 3. Biochim Biophys Acta. 2013. PMID: 23644036 Review.
-
The development of recombinant human serum albumin.Ther Apher. 1998 Nov;2(4):257-62. doi: 10.1111/j.1744-9987.1998.tb00118.x. Ther Apher. 1998. PMID: 10227751 Review.
Cited by
-
Analytical and Structural Evaluation of Recombinant Human Serum Albumin and Fragment F8 for Aptamer-Based Urinary Biomarker Detection.ACS Omega. 2025 Jul 14;10(28):30935-30943. doi: 10.1021/acsomega.5c03518. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727755 Free PMC article.
-
Cibacron Blue F3GA ligand dye-based magnetic silica particles for the albumin purification.Turk J Chem. 2023 Oct 10;47(5):1125-1137. doi: 10.55730/1300-0527.3599. eCollection 2023. Turk J Chem. 2023. PMID: 38173736 Free PMC article.
-
A double built-in containment strategy for production of recombinant proteins in transgenic rice.PLoS One. 2014 Dec 22;9(12):e115459. doi: 10.1371/journal.pone.0115459. eCollection 2014. PLoS One. 2014. PMID: 25531447 Free PMC article.
-
Molecular characterization and efficacy evaluation of a transgenic corn event for insect resistance and glyphosate tolerance.J Zhejiang Univ Sci B. 2018 Aug.;19(8):610-619. doi: 10.1631/jzus.B1700345. J Zhejiang Univ Sci B. 2018. PMID: 30070084 Free PMC article.
-
Generation of insect-resistant and glyphosate-tolerant rice by introduction of a T-DNA containing two Bt insecticidal genes and an EPSPS gene.J Zhejiang Univ Sci B. 2015 Oct;16(10):824-31. doi: 10.1631/jzus.B1500056. J Zhejiang Univ Sci B. 2015. PMID: 26465130 Free PMC article.
References
-
- Belew M, Yan M, Zhang W, Caldwell K. Purification of recombinant human serum albumin (rHSA) produced by genetically modified Pichia pastoris . Separ Sci Technol. 2008;43(11-12):3134–3153. doi: 10.1080/01496390802221857. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

