Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis
- PMID: 24101373
- PMCID: PMC3838581
- DOI: 10.1007/s12265-013-9512-x
Effects of tafamidis on transthyretin stabilization and clinical outcomes in patients with non-Val30Met transthyretin amyloidosis
Abstract
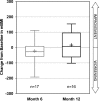
This phase II, open-label, single-treatment arm study evaluated the pharmacodynamics, efficacy, and safety of tafamidis in patients with non-Val30Met transthyretin (TTR) amyloidosis. Twenty-one patients with eight different non-Val30Met mutations received 20 mg QD of tafamidis meglumine for 12 months. The primary outcome, TTR stabilization at Week 6, was achieved in 18 (94.7%) of 19 patients with evaluable data. TTR was stabilized in 100% of patients with non-missing data at Months 6 (n = 18) and 12 (n = 17). Exploratory efficacy measures demonstrated some worsening of neurological function. However, health-related quality of life, cardiac biomarker N-terminal pro-hormone brain natriuretic peptide, echocardiographic parameters, and modified body mass index did not demonstrate clinically relevant worsening during the 12 months of treatment. Tafamidis was well tolerated. In conclusion, our findings suggest that tafamidis 20 mg QD effectively stabilized TTR associated with several non-Val30Met variants.
Trial registration: ClinicalTrials.gov NCT00630864.
Figures



References
-
- Blake CC, Geisow MJ, Swan ID, Rerat C, Rerat B. Structure of human plasma prealbumin at 2–5 A resolution. A preliminary report on the polypeptide chain conformation, quaternary structure and thyroxine binding. Journal of Molecular Biology. 1974;88:1–12. doi: 10.1016/0022-2836(74)90291-5. - DOI - PubMed
-
- Mita S, Maeda S, Shimada K, Araki S. Analyses of prealbumin mRNAs in individuals with familial amyloidotic polyneuropathy. Journal of Biochemistry. 1986;100:1215–1222. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

