The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of Grueneberg ganglion neurons
- PMID: 24101433
- PMCID: PMC11488964
- DOI: 10.1007/s10571-013-9992-x
The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of Grueneberg ganglion neurons
Abstract
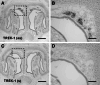
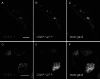
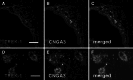
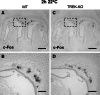
Neurons of the Grueneberg ganglion (GG) residing in the vestibule of the murine nose are activated by cool ambient temperatures. Activation of thermosensory neurons is usually mediated by thermosensitive ion channels of the transient receptor potential (TRP) family. However, there is no evidence for the expression of thermo-TRPs in the GG, suggesting that GG neurons utilize distinct mechanisms for their responsiveness to cool temperatures. In search for proteins that render GG neurons responsive to coolness, we have investigated whether TREK/TRAAK channels may play a role; in heterologous expression systems, these potassium channels have been previously found to close upon exposure to coolness, leading to a membrane depolarization. The results of the present study indicate that the thermosensitive potassium channel TREK-1 is expressed in those GG neurons that are responsive to cool temperatures. Studies analyzing TREK-deficient mice revealed that coolness-evoked responses of GG neurons were clearly attenuated in these animals compared with wild-type conspecifics. These data suggest that TREK-1 channels significantly contribute to the responsiveness of GG neurons to cool temperatures, further supporting the concept that TREK channels serve as thermoreceptors in sensory cells. Moreover, the present findings provide the first evidence of how thermosensory GG neurons are activated by given temperature stimuli in the absence of thermo-TRPs.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
The cyclic nucleotide-gated ion channel CNGA3 contributes to coolness-induced responses of Grueneberg ganglion neurons.Cell Mol Life Sci. 2010 Jun;67(11):1859-69. doi: 10.1007/s00018-010-0296-8. Epub 2010 Feb 18. Cell Mol Life Sci. 2010. PMID: 20165899 Free PMC article.
-
Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells.Neurosci Lett. 2009 Apr 24;454(2):129-33. doi: 10.1016/j.neulet.2009.02.069. Epub 2009 Mar 5. Neurosci Lett. 2009. PMID: 19429069
-
Expression of cGMP signaling elements in the Grueneberg ganglion.Histochem Cell Biol. 2009 Jan;131(1):75-88. doi: 10.1007/s00418-008-0514-8. Epub 2008 Oct 1. Histochem Cell Biol. 2009. PMID: 18830617
-
Ion Channels and Thermosensitivity: TRP, TREK, or Both?Int J Mol Sci. 2019 May 14;20(10):2371. doi: 10.3390/ijms20102371. Int J Mol Sci. 2019. PMID: 31091651 Free PMC article. Review.
-
The Grueneberg ganglion: signal transduction and coding in an olfactory and thermosensory organ involved in the detection of alarm pheromones and predator-secreted kairomones.Cell Tissue Res. 2021 Jan;383(1):535-548. doi: 10.1007/s00441-020-03380-w. Epub 2021 Jan 6. Cell Tissue Res. 2021. PMID: 33404842 Review.
Cited by
-
Are TREK Channels Temperature Sensors?Front Cell Neurosci. 2021 Oct 6;15:744702. doi: 10.3389/fncel.2021.744702. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34690704 Free PMC article. Review.
-
Receptor guanylyl cyclase-G is a novel thermosensory protein activated by cool temperatures.EMBO J. 2015 Feb 3;34(3):294-306. doi: 10.15252/embj.201489652. Epub 2014 Dec 1. EMBO J. 2015. PMID: 25452496 Free PMC article.
-
Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion.Front Neuroanat. 2014 Aug 27;8:87. doi: 10.3389/fnana.2014.00087. eCollection 2014. Front Neuroanat. 2014. PMID: 25221478 Free PMC article.
-
Ion Channel Dysregulation Following Intracerebral Hemorrhage.Neurosci Bull. 2024 Mar;40(3):401-414. doi: 10.1007/s12264-023-01118-6. Epub 2023 Sep 27. Neurosci Bull. 2024. PMID: 37755675 Free PMC article. Review.
-
Magnetic activation of TREK1 triggers stress signalling and regulates neuronal branching in SH-SY5Y cells.Front Med Technol. 2022 Dec 5;4:981421. doi: 10.3389/fmedt.2022.981421. eCollection 2022. Front Med Technol. 2022. PMID: 36545473 Free PMC article.
References
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448:204–208 - PubMed
-
- Brechbühl J, Klaey M, Broillet MC (2008) Grueneberg ganglion cells mediate alarm pheromone detection in mice. Science 321:1092–1095 - PubMed
-
- Colburn RW, Lubin ML, Stone DJ Jr, Wang Y, Lawrence D, D’Andrea MR, Brandt MR, Liu Y, Flores CM, Qin N (2007) Attenuated cold sensitivity in TRPM8 null mice. Neuron 54:379–386 - PubMed
-
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A (2007) TRPM8 is required for cold sensation in mice. Neuron 54:371–378 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

