High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa
- PMID: 24101466
- PMCID: PMC3808598
- DOI: 10.1073/pnas.1311016110
High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa
Abstract
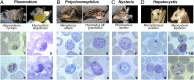
As the only volant mammals, bats are captivating for their high taxonomic diversity, for their vital roles in ecosystems--particularly as pollinators and insectivores--and, more recently, for their important roles in the maintenance and transmission of zoonotic viral diseases. Genome sequences have identified evidence for a striking expansion of and positive selection in gene families associated with immunity. Bats have also been known to be hosts of malaria parasites for over a century, and as hosts, they possess perhaps the most phylogenetically diverse set of hemosporidian genera and species. To provide a molecular framework for the study of these parasites, we surveyed bats in three remote areas of the Upper Guinean forest ecosystem. We detected four distinct genera of hemosporidian parasites: Plasmodium, Polychromophilus, Nycteria, and Hepatocystis. Intriguingly, the two species of Plasmodium in bats fall within the clade of rodent malaria parasites, indicative of multiple host switches across mammalian orders. We show that Nycteria species form a very distinct phylogenetic group and that Hepatocystis parasites display an unusually high diversity and prevalence in epauletted fruit bats. The diversity and high prevalence of novel lineages of chiropteran hemosporidians underscore the exceptional position of bats among all other mammalian hosts of hemosporidian parasites and support hypotheses of pathogen tolerance consistent with the exceptional immunology of bats.
Keywords: Chiroptera; Haemosporida; host–pathogen coevolution; molecular phylogeny; vector-borne disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sutherland CJ, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201(10):1544–1550. - PubMed
-
- Singh BK, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363(9414):1017–1024. - PubMed
-
- Martinsen ES, Perkins SL. In: Malaria Parasites: Comparative Genomics, Evolution and Molecular Biology. Carlton JM, Perkins SL, Deitsch KW, editors. Norfolk, United Kingdom: Caister Academic; 2013. pp. 1–15.
-
- Levine N. The Protozoan Phylum Apicomplexa. Boca Raton, FL: CRC; 1988.
-
- Garnham PCC. Malaria Parasites and Other Haemosporidia. Oxford: Blackwell Scientific; 1966.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

