Humanized mouse model of glucose 6-phosphate dehydrogenase deficiency for in vivo assessment of hemolytic toxicity
- PMID: 24101478
- PMCID: PMC3808620
- DOI: 10.1073/pnas.1310402110
Humanized mouse model of glucose 6-phosphate dehydrogenase deficiency for in vivo assessment of hemolytic toxicity
Abstract
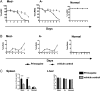
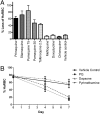
Individuals with glucose 6-phosphate dehydrogenase (G6PD) deficiency are at risk for the development of hemolytic anemia when given 8-aminoquinolines (8-AQs), an important class of antimalarial/antiinfective therapeutics. However, there is no suitable animal model that can predict the clinical hemolytic potential of drugs. We developed and validated a human (hu)RBC-SCID mouse model by giving nonobese diabetic/SCID mice daily transfusions of huRBCs from G6PD-deficient donors. Treatment of SCID mice engrafted with G6PD-deficient huRBCs with primaquine, an 8-AQ, resulted in a dose-dependent selective loss of huRBCs. To validate the specificity of this model, we tested known nonhemolytic antimalarial drugs: mefloquine, chloroquine, doxycycline, and pyrimethamine. No significant loss of G6PD-deficient huRBCs was observed. Treatment with drugs known to cause hemolytic toxicity (pamaquine, sitamaquine, tafenoquine, and dapsone) resulted in loss of G6PD-deficient huRBCs comparable to primaquine. This mouse model provides an important tool to test drugs for their potential to cause hemolytic toxicity in G6PD-deficient populations.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Nkhoma ET, Poole C, Vannappagari V, Hall SA, Beutler E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol Dis. 2009;42(3):267–278. - PubMed
-
- Beutler E, Duparc S. G6PD Deficiency Working Group Glucose-6-phosphate dehydrogenase deficiency and antimalarial drug development. Am J Trop Med Hyg. 2007;77(4):779–789. - PubMed
-
- Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39(9):1336–1345. - PubMed
-
- Brueckner RP, Ohrt C, Baird JK, Milhous WK. 8-Aminoquinolines. In: Rosenthal P, editor. Anti-malarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Totowa, NJ: Humana; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

