Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6
- PMID: 24101490
- PMCID: PMC3808642
- DOI: 10.1073/pnas.1314017110
Implication of the anti-inflammatory bioactive lipid prostaglandin D2-glycerol ester in the control of macrophage activation and inflammation by ABHD6
Abstract
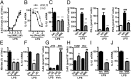
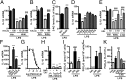
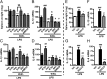
Proinflammatory macrophages are key mediators in several pathologies; thus, controlling their activation is necessary. The endocannabinoid system is implicated in various inflammatory processes. Here we show that in macrophages, the newly characterized enzyme α/β-hydrolase domain 6 (ABHD6) controls 2-arachidonoylglycerol (2-AG) levels and thus its pharmacological effects. Furthermore, we characterize a unique pathway mediating the effects of 2-AG through its oxygenation by cyclooxygenase-2 to give rise to the anti-inflammatory prostaglandin D2-glycerol ester (PGD2-G). Pharmacological blockade of cyclooxygenase-2 or of prostaglandin D synthase prevented the effects of increasing 2-AG levels by ABHD6 inhibition in vitro, as well as the 2-AG-induced increase in PGD2-G levels. Together, our data demonstrate the physiological relevance of the interaction between the endocannabinoid and prostanoid systems. Moreover, we show that ABHD6 inhibition in vivo allows for fine-tuning of 2-AG levels in mice, therefore reducing lipopolysaccharide-induced inflammation, without the characteristic central side effects of strong increases in 2-AG levels obtained following monoacylglycerol lipase inhibition. In addition, administration of PGD2-G reduces lipopolysaccharide-induced inflammation in mice, thus confirming the biological relevance of this 2-AG metabolite. This points to ABHD6 as an interesting therapeutic target that should be relevant in treating inflammation-related conditions, and proposes PGD2-G as a bioactive lipid with potential anti-inflammatory properties in vivo.
Keywords: COX-2; FAAH; anandamide; glyceryl prostaglandin; prostaglandin synthase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
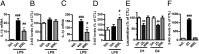
References
-
- Valledor AF, Comalada M, Santamaría-Babi LF, Lloberas J, Celada A. Macrophage proinflammatory activation and deactivation: A question of balance. Adv Immunol. 2010;108:1–20. - PubMed
-
- Alhouayek M, Lambert DM, Delzenne NM, Cani PD, Muccioli GG. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25(8):2711–2721. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

