Network model of top-down influences on local gain and contextual interactions in visual cortex
- PMID: 24101495
- PMCID: PMC3808648
- DOI: 10.1073/pnas.1317019110
Network model of top-down influences on local gain and contextual interactions in visual cortex
Abstract
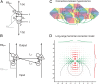
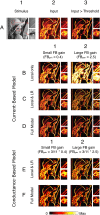
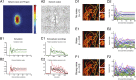
The visual system uses continuity as a cue for grouping oriented line segments that define object boundaries in complex visual scenes. Many studies support the idea that long-range intrinsic horizontal connections in early visual cortex contribute to this grouping. Top-down influences in primary visual cortex (V1) play an important role in the processes of contour integration and perceptual saliency, with contour-related responses being task dependent. This suggests an interaction between recurrent inputs to V1 and intrinsic connections within V1 that enables V1 neurons to respond differently under different conditions. We created a network model that simulates parametrically the control of local gain by hypothetical top-down modification of local recurrence. These local gain changes, as a consequence of network dynamics in our model, enable modulation of contextual interactions in a task-dependent manner. Our model displays contour-related facilitation of neuronal responses and differential foreground vs. background responses over the neuronal ensemble, accounting for the perceptual pop-out of salient contours. It quantitatively reproduces the results of single-unit recording experiments in V1, highlighting salient contours and replicating the time course of contextual influences. We show by means of phase-plane analysis that the model operates stably even in the presence of large inputs. Our model shows how a simple form of top-down modulation of the effective connectivity of intrinsic cortical connections among biophysically realistic neurons can account for some of the response changes seen in perceptual learning and task switching.
Keywords: feedback; gating; long-range horizontal connections; reentrant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Circuits for local and global signal integration in primary visual cortex.J Neurosci. 2002 Oct 1;22(19):8633-46. doi: 10.1523/JNEUROSCI.22-19-08633.2002. J Neurosci. 2002. PMID: 12351737 Free PMC article.
-
A model of contextual interactions and contour detection in primary visual cortex.Neural Netw. 2004 Jun-Jul;17(5-6):719-35. doi: 10.1016/j.neunet.2004.03.007. Neural Netw. 2004. PMID: 15288894
-
The role of feedback in shaping the extra-classical receptive field of cortical neurons: a recurrent network model.J Neurosci. 2006 Sep 6;26(36):9117-29. doi: 10.1523/JNEUROSCI.1253-06.2006. J Neurosci. 2006. PMID: 16957068 Free PMC article.
-
Neural circuit models for computations in early visual cortex.Curr Opin Neurobiol. 2011 Oct;21(5):808-15. doi: 10.1016/j.conb.2011.07.005. Epub 2011 Aug 26. Curr Opin Neurobiol. 2011. PMID: 21873046 Review.
-
The dynamics of visual responses in the primary visual cortex.Prog Brain Res. 2007;165:21-32. doi: 10.1016/S0079-6123(06)65003-6. Prog Brain Res. 2007. PMID: 17925238 Review.
Cited by
-
Coordination Dynamics in Cognitive Neuroscience.Front Neurosci. 2016 Sep 15;10:397. doi: 10.3389/fnins.2016.00397. eCollection 2016. Front Neurosci. 2016. PMID: 27695395 Free PMC article. Review.
-
Operating in a Reverberating Regime Enables Rapid Tuning of Network States to Task Requirements.Front Syst Neurosci. 2018 Nov 6;12:55. doi: 10.3389/fnsys.2018.00055. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30459567 Free PMC article.
-
Contextual Integration in Cortical and Convolutional Neural Networks.Front Comput Neurosci. 2020 Apr 23;14:31. doi: 10.3389/fncom.2020.00031. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32390818 Free PMC article.
-
Adult cortical plasticity studied with chronically implanted electrode arrays.J Neurosci. 2015 Feb 11;35(6):2778-90. doi: 10.1523/JNEUROSCI.3579-14.2015. J Neurosci. 2015. PMID: 25673865 Free PMC article.
-
Beyond Rehabilitation of Acuity, Ocular Alignment, and Binocularity in Infantile Strabismus.Front Syst Neurosci. 2018 Jul 18;12:29. doi: 10.3389/fnsys.2018.00029. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30072876 Free PMC article.
References
-
- Wertheimer M. Untersuchung zur Lehre von der Gestalt. Psychol Forsch. 1923;4(1):301–350.
-
- Li W, Piëch V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50(6):951–962. - PubMed
-
- Ernst UA, Mandon S, Pawelzik KR, Kreiter AK. How ideal do macaque monkeys integrate contours? Neurocomputing. 2004;58–60:971–977.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

