Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms
- PMID: 24101505
- PMCID: PMC3808666
- DOI: 10.1073/pnas.1308987110
Arabidopsis CRY2 and ZTL mediate blue-light regulation of the transcription factor CIB1 by distinct mechanisms
Abstract
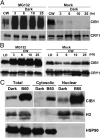
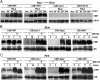
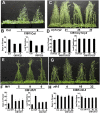
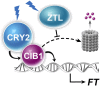
Plants possess multiple photoreceptors to mediate light regulation of growth and development, but it is not well understood how different photoreceptors coordinate their actions to jointly regulate developmental responses, such as flowering time. In Arabidopsis, the photoexcited cryptochrome 2 interacts with the transcription factor CRYPTOCHROME-INTERACTING basic helix-loop-helix 1 (CIB1) to activate transcription and floral initiation. We show that the CIB1 protein expression is regulated by blue light; CIB1 is highly expressed in plants exposed to blue light, but levels of the CIB1 protein decreases in the absence of blue light. We demonstrate that CIB1 is degraded by the 26S proteasome and that blue light suppresses CIB1 degradation. Surprisingly, although cryptochrome 2 physically interacts with CIB1 in response to blue light, it is not the photoreceptor mediating blue-light suppression of CIB1 degradation. Instead, two of the three light-oxygen-voltage (LOV)-domain photoreceptors, ZEITLUPE and LOV KELCH PROTEIN 2, but not FLAVIN-BINDING KELCH REPEAT 1, are required for the function and blue-light suppression of degradation of CIB1. These results support the hypothesis that the evolutionarily unrelated blue-light receptors, cryptochrome and LOV-domain F-box proteins, mediate blue-light regulation of the same transcription factor by distinct mechanisms.
Keywords: gene expression; photomorphogenesis; protein degradation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cashmore AR. Cryptochromes: Enabling plants and animals to determine circadian time. Cell. 2003;114(5):537–543. - PubMed
-
- Sancar A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. Chem Rev. 2003;103(6):2203–2237. - PubMed
-
- Chaves I, et al. The cryptochromes: Blue light photoreceptors in plants and animals. Annu Rev Plant Biol. 2011;62:335–364. - PubMed
-
- Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366(6451):162–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

