Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity
- PMID: 24101524
- PMCID: PMC3808637
- DOI: 10.1073/pnas.1304266110
Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity
Abstract
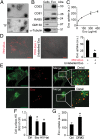
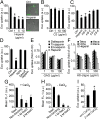
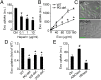
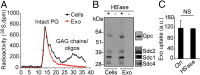
Extracellular vesicle (EV)-mediated intercellular transfer of signaling proteins and nucleic acids has recently been implicated in the development of cancer and other pathological conditions; however, the mechanism of EV uptake and how this may be targeted remain as important questions. Here, we provide evidence that heparan sulfate (HS) proteoglycans (PGs; HSPGs) function as internalizing receptors of cancer cell-derived EVs with exosome-like characteristics. Internalized exosomes colocalized with cell-surface HSPGs of the syndecan and glypican type, and exosome uptake was specifically inhibited by free HS chains, whereas closely related chondroitin sulfate had no effect. By using several cell mutants, we provide genetic evidence of a receptor function of HSPG in exosome uptake, which was dependent on intact HS, specifically on the 2-O and N-sulfation groups. Further, enzymatic depletion of cell-surface HSPG or pharmacological inhibition of endogenous PG biosynthesis by xyloside significantly attenuated exosome uptake. We provide biochemical evidence that HSPGs are sorted to and associate with exosomes; however, exosome-associated HSPGs appear to have no direct role in exosome internalization. On a functional level, exosome-induced ERK1/2 signaling activation was attenuated in PG-deficient mutant cells as well as in WT cells treated with xyloside. Importantly, exosome-mediated stimulation of cancer cell migration was significantly reduced in PG-deficient mutant cells, or by treatment of WT cells with heparin or xyloside. We conclude that cancer cell-derived exosomes use HSPGs for their internalization and functional activity, which significantly extends the emerging role of HSPGs as key receptors of macromolecular cargo.
Keywords: endocytosis; glioma; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–1495. - PubMed
-
- Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. - PubMed
-
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

