Radiotracer imaging of peripheral vascular disease
- PMID: 24101686
- PMCID: PMC6503532
- DOI: 10.2967/jnumed.112.115105
Radiotracer imaging of peripheral vascular disease
Abstract
Peripheral vascular disease (PVD) is an atherosclerotic disease affecting the lower extremities, resulting in skeletal muscle ischemia, intermittent claudication, and, in more severe stages of disease, limb amputation and death. The evaluation of therapy in this patient population can be challenging, as the standard clinical indices are insensitive to assessment of regional alterations in skeletal muscle physiology. Radiotracer imaging of the lower extremities with techniques such as PET and SPECT can provide a noninvasive quantitative technique for the evaluation of the pathophysiology associated with PVD and may complement clinical indices and other imaging approaches. This review discusses the progress in radiotracer-based evaluation of PVD and highlights recent advancements in molecular imaging with potential for clinical application.
Keywords: PET; SPECT; angiogenesis; perfusion; peripheral vascular disease.
Figures
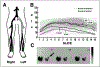
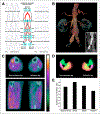

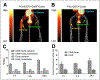

References
-
- Peach G, Griffin M, Jones KG, Thompson MM, Hinchliffe RJ. Diagnosis and management of peripheral arterial disease. BMJ. 2012;345:e5208. - PubMed
-
- Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population >=40 years of age with and without diabetes: 1999-2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. - PubMed
-
- Hooi JD, Stoffers HE, Kester AD, et al. Risk factors and cardiovascular diseases associated with asymptomatic peripheral arterial occlusive disease. The Limburg PAOD study. Peripheral Arterial Occlusive Disease. Scand J Prim Health Care. 1998;16:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
