CD4+ T cells drive goblet cell depletion during Citrobacter rodentium infection
- PMID: 24101690
- PMCID: PMC3837981
- DOI: 10.1128/IAI.00655-13
CD4+ T cells drive goblet cell depletion during Citrobacter rodentium infection
Abstract
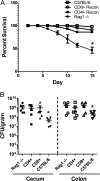
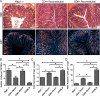
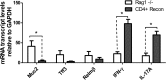
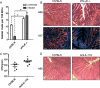
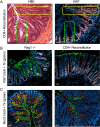
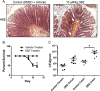
Both idiopathic and infectious forms of colitis disrupt normal intestinal epithelial cell (IEC) proliferation and differentiation, although the mechanisms involved remain unclear. Recently, we demonstrated that infection by the attaching and effacing murine pathogen Citrobacter rodentium leads to a significant reduction in colonic goblet cell numbers (goblet cell depletion). This pathology depends on T and/or B cells, as Rag1(-/-) mice do not suffer this depletion during infection, instead suffering high mortality rates. To address the immune mechanisms involved, we reconstituted Rag(-/-) mice with either CD4(+) or CD8(+) T cells. Both T cell subsets increased Rag1(-/-) mouse survival during infection, with mice that received CD8(+) T cells developing colonic ulcers but not goblet cell depletion. In contrast, mice that received CD4(+) T cells showed goblet cell depletion in concert with exaggerated IEC proliferation. To define the possible involvement of T cell-derived cytokines, we infected gamma interferon receptor gene knockout (IFN-γR(-/-)) mice and wild-type mice given interleukin 17A (IL-17A) neutralizing antibodies and found that IFN-γ signaling was required for both goblet cell depletion and increased IEC proliferation. Immunostaining revealed that C. rodentium cells preferentially localized to nonhyperplastic crypts containing numerous goblet cells, whereas hyperplastic, goblet cell-depleted crypts appeared protected from infection. To address whether goblet cell depletion benefits the C. rodentium-infected host, we increased goblet cell numbers using the γ-secretase inhibitor dibenzazepine (DBZ), which resulted in greatly increased pathogen burdens and mortality rates. These results demonstrate that goblet cell depletion reflects host immunomodulation of IEC homeostasis and reflects a novel host defense mechanism against mucosal-adherent pathogens.
Figures


Similar articles
-
Goblet Cell Derived RELM-β Recruits CD4+ T Cells during Infectious Colitis to Promote Protective Intestinal Epithelial Cell Proliferation.PLoS Pathog. 2015 Aug 18;11(8):e1005108. doi: 10.1371/journal.ppat.1005108. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26285214 Free PMC article.
-
Intestinal epithelium-specific MyD88 signaling impacts host susceptibility to infectious colitis by promoting protective goblet cell and antimicrobial responses.Infect Immun. 2014 Sep;82(9):3753-63. doi: 10.1128/IAI.02045-14. Epub 2014 Jun 23. Infect Immun. 2014. PMID: 24958710 Free PMC article.
-
CD4+-T-cell effector functions and costimulatory requirements essential for surviving mucosal infection with Citrobacter rodentium.Infect Immun. 2006 Jan;74(1):673-81. doi: 10.1128/IAI.74.1.673-681.2006. Infect Immun. 2006. PMID: 16369024 Free PMC article.
-
Citrobacter rodentium-host-microbiota interactions: immunity, bioenergetics and metabolism.Nat Rev Microbiol. 2019 Nov;17(11):701-715. doi: 10.1038/s41579-019-0252-z. Epub 2019 Sep 20. Nat Rev Microbiol. 2019. PMID: 31541196 Review.
-
T cell subsets and environmental factors in Citrobacter rodentium infection.Curr Opin Microbiol. 2021 Oct;63:92-97. doi: 10.1016/j.mib.2021.06.006. Epub 2021 Jul 21. Curr Opin Microbiol. 2021. PMID: 34298480 Review.
Cited by
-
The cell surface receptor Slamf6 modulates innate immune responses during Citrobacter rodentium-induced colitis.Int Immunol. 2015 Sep;27(9):447-57. doi: 10.1093/intimm/dxv029. Epub 2015 May 8. Int Immunol. 2015. PMID: 25957267 Free PMC article.
-
A histomorphometric classification system for normal and inflamed mouse duodenum-Quali-quantitative approach.Int J Exp Pathol. 2018 Aug;99(4):189-198. doi: 10.1111/iep.12286. Epub 2018 Sep 2. Int J Exp Pathol. 2018. PMID: 30175413 Free PMC article.
-
Effects of Yacon on Colonic IFN-γ and Goblet Cells of 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis Mouse Model.Iran Biomed J. 2020 Sep;24(5):281-7. doi: 10.29252/ibj.24.5.276. Epub 2020 Jan 20. Iran Biomed J. 2020. PMID: 32429633 Free PMC article.
-
Milk Fat Globule Membrane Supplementation in Formula Modulates the Neonatal Gut Microbiome and Normalizes Intestinal Development.Sci Rep. 2017 Mar 28;7:45274. doi: 10.1038/srep45274. Sci Rep. 2017. PMID: 28349941 Free PMC article.
-
Epithelial Histone Deacetylase 3 Instructs Intestinal Immunity by Coordinating Local Lymphocyte Activation.Cell Rep. 2017 May 9;19(6):1165-1175. doi: 10.1016/j.celrep.2017.04.046. Cell Rep. 2017. PMID: 28494866 Free PMC article.
References
-
- Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. 2012. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 8:36–45 - PubMed
-
- Goto Y, Kiyono H. 2012. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol. Rev. 245:147–163 - PubMed
-
- Karam SM. 1999. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 4:D286–D298 - PubMed
-
- Allen A, Hutton DA, Pearson JP. 1998. The MUC2 gene product: a human intestinal mucin. Int. J. Biochem. Cell Biol. 30:797–801 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

