Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs
- PMID: 24101727
- PMCID: PMC3860313
- DOI: 10.1242/jcs.136549
Regulation of endoplasmic reticulum Ca(2+) oscillations in mammalian eggs
Abstract
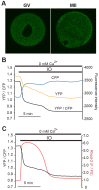
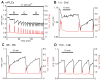
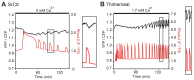
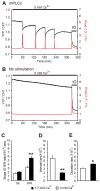
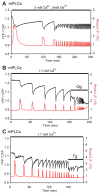
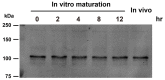
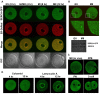
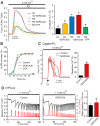
Changes in the intracellular concentration of free calcium ([Ca(2+)]i) regulate diverse cellular processes including fertilization. In mammalian eggs, the [Ca(2+)]i changes induced by the sperm unfold in a pattern of periodical rises, also known as [Ca(2+)]i oscillations. The source of Ca(2+) during oscillations is the endoplasmic reticulum ([Ca(2+)]ER), but it is presently unknown how [Ca(2+)]ER is regulated. Here, we show using mouse eggs that [Ca(2+)]i oscillations induced by a variety of agonists, including PLCζ, SrCl2 and thimerosal, provoke simultaneous but opposite changes in [Ca(2+)]ER and cause differential effects on the refilling and overall load of [Ca(2+)]ER. We also found that Ca(2+) influx is required to refill [Ca(2+)]ER, because the loss of [Ca(2+)]ER was accelerated in medium devoid of Ca(2+). Pharmacological inactivation of the function of the mitochondria and of the Ca(2+)-ATPase pumps PMCA and SERCA altered the pattern of oscillations and abruptly reduced [Ca(2+)]ER, especially after inactivation of mitochondria and SERCA functions. We also examined the expression of SERCA2b protein and found that it was expressed throughout oocyte maturation and attained a conspicuous cortical cluster organization in mature eggs. We show that its overexpression reduces the duration of inositol-1,4,5-trisphosphate-induced [Ca(2+)]i rises, promotes initiation of oscillations and enhances refilling of [Ca(2+)]ER. Collectively, our results provide novel insights on the regulation of [Ca(2+)]ER oscillations, which underlie the unique Ca(2+)-signalling system that activates the developmental program in mammalian eggs.
Keywords: Ca2+ oscillations; Egg activation; Endoplasmic reticulum; Fertilization; Oocyte maturation; SERCA.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

