Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment
- PMID: 24101862
- PMCID: PMC3790833
- DOI: 10.2147/DDDT.S44706
Amniotic fluid stem cells morph into a cardiovascular lineage: analysis of a chemically induced cardiac and vascular commitment
Abstract
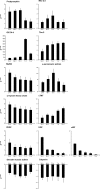
Mouse embryonic stem cells were previously observed along with mesenchymal stem cells from different sources, after being treated with a mixed ester of hyaluronan with butyric and retinoic acids, to show a significant increase in the yield of cardiogenic and vascular differentiated elements. The aim of the present study was to determine if stem cells derived from primitive fetal cells present in human amniotic fluid (hAFSCs) and cultured in the presence of a mixture of hyaluronic (HA), butyric (BU), and retinoic (RA) acids show a higher yield of differentiation toward the cardiovascular phenotype as compared with untreated cells. During the differentiation process elicited by exposure to HA + BU + RA, genes controlling pluripotency and plasticity of stem cells, such as Sox2, Nanog, and Oct4, were significantly downregulated at the transcriptional level. At this point, a significant increase in expression of genes controlling the appearance of cardiogenic and vascular lineages in HA + BU + RA-treated cells was observed. The protein expression levels typical of cardiac and vascular phenotypes, evaluated by Western blotting, immunofluorescence, and flow cytometry, were higher in hAFSCs cultured in the presence of HA + BU + RA, as compared with untreated control cells. Appearance of the cardiac phenotype was further inferred by ultrastructural analysis using transmission and scanning electron microscopy. These results demonstrate that a mixture of HA + BU + RA significantly increased the yield of elements committed toward cardiac and vascular phenotypes, confirming what we have previously observed in other cellular types.
Keywords: GATA-4; Nkx-2.5; butyric acid; fetal cells; human amniotic fluid; hyaluronic acid; prodynorphin; retinoic acid; von Willebrand factor.
Figures





References
-
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95(1):9–20. - PubMed
-
- Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Res Cardiol. 2005;100(6):471–481. - PubMed
-
- De Coppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–106. - PubMed
-
- Sartore S, Lenzi M, Angelini A, et al. Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. Eur J Cardiothorac Surg. 2005;28(5):677–684. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

