The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system
- PMID: 24102106
- PMCID: PMC3874693
- DOI: 10.1111/bph.12407
The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system
Abstract
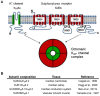
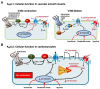
ATP-sensitive potassium channels (K(ATP)) are widely distributed and present in a number of tissues including muscle, pancreatic beta cells and the brain. Their activity is regulated by adenine nucleotides, characteristically being activated by falling ATP and rising ADP levels. Thus, they link cellular metabolism with membrane excitability. Recent studies using genetically modified mice and genomic studies in patients have implicated K(ATP) channels in a number of physiological and pathological processes. In this review, we focus on their role in cellular function and protection particularly in the cardiovascular system.
Keywords: ATP-sensitive potassium channel; cardiac myocyte; pathophysiology; physiology; smooth muscle.
© 2013 The British Pharmacological Society.
Figures
References
-
- Aguilar Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

