Global clinical response in Cushing's syndrome patients treated with mifepristone
- PMID: 24102404
- PMCID: PMC4255292
- DOI: 10.1111/cen.12332
Global clinical response in Cushing's syndrome patients treated with mifepristone
Abstract
Objective: Mifepristone, a glucocorticoid receptor antagonist, improves clinical status in patients with Cushing's syndrome (CS). We examined the pattern, reliability and correlates of global clinical response (GCR) assessments during a 6-month clinical trial of mifepristone in CS.
Design: Post hoc analysis of secondary end-point data from a 24-week multicentre, open-label trial of mifepristone (300-1200 mg daily) in CS. Intraclass correlation coefficient (ICC) was used to examine rater concordance, and drivers of clinical improvement were determined by multivariate regression analysis.
Patients: Forty-six adult patients with refractory CS along with diabetes mellitus type 2 or impaired glucose tolerance, and/or a diagnosis of hypertension.
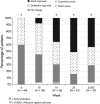
Measurements: Global clinical assessment made by three independent reviewers using a three-point ordinal scale (+1 = improvement; 0 = no change; -1 = worsening) based on eight broad clinical categories including glucose control, lipids, blood pressure, body composition, clinical appearance, strength, psychiatric/cognitive symptoms and quality of life at Weeks 6, 10, 16, and 24.
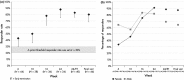
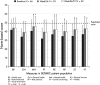
Results: Positive GCR increased progressively over time with 88% of patients having improved at Week 24 (P < 0·001). The full concordance among reviewers occurred in 76·6% of evaluations resulting in an ICC of 0·652 (P < 0·001). Changes in body weight (P < 0·0001), diastolic blood pressure (P < 0·0001), two-hour postoral glucose challenge glucose concentration (P = 0·0003), and Cushingoid appearance (P = 0·022) were strong correlates of GCR.
Conclusions: Mifepristone treatment for CS results in progressive clinical improvement. Overall agreement among clinical reviewers was substantial and determinants of positive GCR included change in weight, blood pressure, glucose levels and appearance.
© 2013 The Authors. Clinical Endocrinology published by John Wiley & Sons Ltd.
Figures
References
-
- Shimon I, Ram Z, Cohen ZR, et al. Transsphenoidal surgery for Cushing’s disease: endocrinological follow-up monitoring of 82 patients. Neurosurgery. 2002;51:57–61. - PubMed
-
- Hammer GD, Tyrrell JB, Lamborn KR, et al. Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. Journal of Clinical Endocrinology and Metabolism. 2004;89:6348–6357. - PubMed
-
- Atkinson AB, Kennedy A, Wiggam MI, et al. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clinical Endocrinology. 2005;63:549–559. - PubMed
-
- Tritos NA. Biller BMK. Advances in medical therapies for Cushing’s syndrome. Discovery Medicine. 2012;13:171–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

