Differential IFN-γ production by adult and neonatal blood CD56+ natural killer (NK) and NK-like-T cells in response to Trypanosoma cruzi and IL-15
- PMID: 24102464
- PMCID: PMC4285850
- DOI: 10.1111/pim.12077
Differential IFN-γ production by adult and neonatal blood CD56+ natural killer (NK) and NK-like-T cells in response to Trypanosoma cruzi and IL-15
Abstract
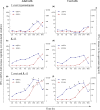
Early interferon-gamma (IFN-γ) release by innate cells is critical to direct type 1 immune response able to control intracellular pathogens like Trypanosoma cruzi. Although CD56(bright) natural killer (NK) cells are reported to be potent early IFN-γ producers, other CD56(+) cells like CD56(dim) NK cells and NK-like T cells have recently been shown to also release IFN-γ. We have here studied the contribution of each CD56(+) lymphocyte populations in early IFN-γ production in both adults and neonates. On this purpose, we analysed the kinetics of IFN-γ production by RT-PCR, ELISA and flow cytometry from 2 h onwards after T. cruzi and IL-15 stimulation and sought for the responding CD56(+) cells. CD56(bright) and CD56(dim) CD16(-) NK cells were the more potent IFN-γ early producers in response to IL-15 and parasites in adults and neonates. In both age groups, the majority of IFN-γ producing cells were NK cells. However, on the contrary to neonates, CD3(+) CD56(+) NK-like T cells and CD3(+) CD56(-) 'classical' T cells also contributed to early IFN-γ production in adults. Altogether, our results support that whereas NK cells responded almost similarly in neonates and adults, cord blood innate CD56(+) and CD56(-) T cells displayed major quantitative and qualitative defects that could contribute to the well-known neonatal immune immaturity.
Keywords: Interferon-gamma; T cell; Trypanosoma cruzi; human neonate and adult; innate immunity; natural killer cell.
© 2013 The Authors. Parasite Immunology published by John Wiley & Sons Ltd.
Figures


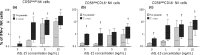

References
-
- Torrico F, Heremans H, Rivera MT, Van Marck E, Billiau A. Carlier Y. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J Immunol. 1991;146:3626–3632. - PubMed
-
- Shtrichman R. Samuel CE. The role of gamma interferon in antimicrobial immunity. Curr Opin Microbiol. 2001;4:251–259. - PubMed
-
- Cooper MA, Fehniger TA. Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. - PubMed
-
- Vitale M, Caruso A, Licenziati S, et al. Differential production of IFN-gamma, analyzed at the single-cell level, by specific subsets of human NK and T cells from healthy and HIV(+) subjects. Cytometry. 2000;39:189–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

