Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice
- PMID: 24102495
- PMCID: PMC4307027
- DOI: 10.1111/pim.12078
Neutrophils have a protective role during early stages of Leishmania amazonensis infection in BALB/c mice
Abstract
Neutrophils are involved in the early stages of immune responses to pathogens. Here, we investigated the role of neutrophils during the establishment of Leishmania amazonensis infection in BALB/c and C57BL/6 mice. First, we showed an accumulation of neutrophils between 6 and 24 h post-infection, followed by a reduction in neutrophil numbers after 72 h. Next, we depleted neutrophils prior to infection using RB6-8C5 or 1A8 mAb. Neutrophil depletion led to faster lesion development, increased parasite numbers and higher arginase activity during the first week of infection in BALB/c mice, but not in C57BL/6 mice. Increased susceptibility was accompanied by augmented levels of anti-L. amazonensis IgG and increased production of IL-10 and IL-17. Because IL-10 is a mediator of susceptibility to Leishmania infection, we blocked IL-10 signalling in neutrophil-depleted mice using anti-IL-10R. Interestingly, inhibition of IL-10 signalling abrogated the increase in parasite loads observed in neutrophil-depleted mice, suggesting that parasite proliferation is at least partially mediated by IL-10. Additionally, we tested the effect of IL-17 in inflammatory macrophages and observed that IL-17 increased arginase activity and favoured parasite growth. Taken together, our data indicate that neutrophils control parasite numbers and limit lesion development during the first week of infection in BALB/c mice.
Keywords: IL-10; Leishmania amazonensis; immune response; neutrophils.
© 2013 John Wiley & Sons Ltd.
Figures



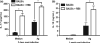


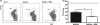

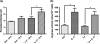
References
-
- Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. - PubMed
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol. 2003;171:6052–6058. - PubMed
-
- Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing pre-stored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. - PubMed
-
- Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

