Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation
- PMID: 24102808
- PMCID: PMC4161737
- DOI: 10.1111/ajt.12433
Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation
Abstract
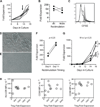
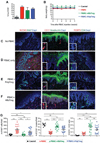
Regulatory T cell (Treg) therapy has the potential to induce transplantation tolerance so that immunosuppression and associated morbidity can be minimized. Alloantigen-reactive Tregs (arTregs) are more effective at preventing graft rejection than polyclonally expanded Tregs (PolyTregs) in murine models. We have developed a manufacturing process to expand human arTregs in short-term cultures using good manufacturing practice-compliant reagents. This process uses CD40L-activated allogeneic B cells to selectively expand arTregs followed by polyclonal restimulation to increase yield. Tregs expanded 100- to 1600-fold were highly alloantigen reactive and expressed the phenotype of stable Tregs. The alloantigen-expanded Tregs had a diverse TCR repertoire. They were more potent than PolyTregs in vitro and more effective at controlling allograft injuries in vivo in a humanized mouse model.
Keywords: Cellular therapy; clinical application; regulatory T cells; tolerance induction.
© Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors of this manuscript have conflicts of interests to disclose as described by the
Figures




References
-
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. - PubMed
-
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. - PubMed
-
- Waldmann H, Adams E, Cobbold S. Reprogramming the immune system: Co-receptor blockade as a paradigm for harnessing tolerance mechanisms. Immunol Rev. 2008;223:361–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

