17β-Estradiol alters oxidative stress response protein expression and oxidative damage in the uterus
- PMID: 24103313
- PMCID: PMC3900311
- DOI: 10.1016/j.mce.2013.09.023
17β-Estradiol alters oxidative stress response protein expression and oxidative damage in the uterus
Abstract
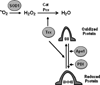
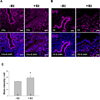
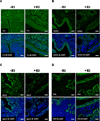
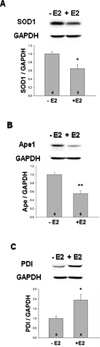
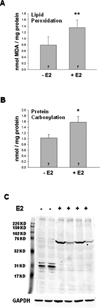
The steroid hormone 17β-estradiol (E2) has profound effects on the uterus. However, with the E2-induced increase in uterine cell proliferation and metabolism comes increased production of reactive oxygen species (ROS). We examined the expression of an interactive network of oxidative stress response proteins including thioredoxin (Trx), Cu/Zn superoxide dismutase (SOD1), apurinic endonuclease (Ape1), and protein disulfide isomerase (PDI). We demonstrated that treatment of ovariectomized C57BL/6J female mice with E2 increased the mRNA and protein levels of Trx, but decreased SOD1 and Ape1 mRNA and protein expression. In contrast, E2 treatment increased PDI protein levels but had no effect on PDI transcript levels. Interestingly, E2 treatment also increased two markers of cellular damage, lipid peroxidation and protein carbonylation. Our studies suggest that the decreased expression of SOD1 and Ape1 caused by E2 treatment may in the long term result in disruption of ROS regulation and play a role in endometrial carcinogenesis.
Keywords: Apurinic endonuclease; Cu/Zn superoxide dismutase; Estrogen receptor ɑ; Oxidative stress; Protein disulfide isomerase; Thioredoxin.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures

Similar articles
-
17β-Estradiol alters oxidative damage and oxidative stress response protein expression in the mouse mammary gland.Mol Cell Endocrinol. 2016 May 5;426:11-21. doi: 10.1016/j.mce.2016.02.007. Epub 2016 Feb 9. Mol Cell Endocrinol. 2016. PMID: 26872614 Free PMC article.
-
17β-estradiol increases expression of the oxidative stress response and DNA repair protein apurinic endonuclease (Ape1) in the cerebral cortex of female mice following hypoxia.J Steroid Biochem Mol Biol. 2013 Nov;138:410-20. doi: 10.1016/j.jsbmb.2013.07.007. Epub 2013 Jul 29. J Steroid Biochem Mol Biol. 2013. PMID: 23907014 Free PMC article.
-
Estradiol regulates the thioredoxin antioxidant system in the mouse uterus.Endocrinology. 2004 Dec;145(12):5485-92. doi: 10.1210/en.2004-0471. Epub 2004 Sep 2. Endocrinology. 2004. PMID: 15345672
-
17beta-estradiol induces protein thiol/disulfide oxidoreductases and protects cultured bovine aortic endothelial cells from oxidative stress.Eur J Endocrinol. 1999 Jun;140(6):608-13. doi: 10.1530/eje.0.1400608. Eur J Endocrinol. 1999. PMID: 10366417
-
17β-Estradiol-mediated increase in Cu/Zn superoxide dismutase expression in the brain: a mechanism to protect neurons from ischemia.J Steroid Biochem Mol Biol. 2011 Nov;127(3-5):382-9. doi: 10.1016/j.jsbmb.2011.06.008. Epub 2011 Jun 17. J Steroid Biochem Mol Biol. 2011. PMID: 21704159 Free PMC article.
Cited by
-
17β-Estradiol alters oxidative damage and oxidative stress response protein expression in the mouse mammary gland.Mol Cell Endocrinol. 2016 May 5;426:11-21. doi: 10.1016/j.mce.2016.02.007. Epub 2016 Feb 9. Mol Cell Endocrinol. 2016. PMID: 26872614 Free PMC article.
-
The synergistic antibacterial activity and mechanism of colistin-oxethazaine combination against gram-negative pathogens.Front Pharmacol. 2024 Mar 21;15:1363441. doi: 10.3389/fphar.2024.1363441. eCollection 2024. Front Pharmacol. 2024. PMID: 38576480 Free PMC article.
-
Effect of monosodium glutamate on serum sex hormones and uterine histology in female rats along with its molecular docking and in-silico toxicity.Heliyon. 2022 Oct 5;8(10):e10967. doi: 10.1016/j.heliyon.2022.e10967. eCollection 2022 Oct. Heliyon. 2022. PMID: 36237979 Free PMC article.
-
NaCl pretreatment attenuates H.pylori-induced DNA damage and exacerbates proliferation of gastric epithelial cells (GES-1).Infect Agent Cancer. 2015 Mar 1;10:8. doi: 10.1186/s13027-015-0003-3. eCollection 2015. Infect Agent Cancer. 2015. PMID: 25859277 Free PMC article.
-
Estradiol Regulates Txnip and Prevents Intermittent Hypoxia-Induced Vascular Injury.Sci Rep. 2017 Sep 4;7(1):10318. doi: 10.1038/s41598-017-10442-7. Sci Rep. 2017. PMID: 28871193 Free PMC article.
References
-
- Arner ES, Holmgren A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006;16:420–426. - PubMed
-
- Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–6109. - PubMed
-
- Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 1995;9:1441–1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

