Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure
- PMID: 24103426
- PMCID: PMC3851868
- DOI: 10.1186/1476-4598-12-118
Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure
Abstract
Background: Extracellular vesicle (EV) trafficking is a fundamental cellular process that occurs in cells and is required for different aspects of pathophysiology. EV trafficking leads to changes in cellular function including apoptosis, angiogenesis and proliferation required for increased tumor formation.
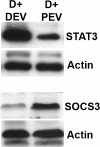
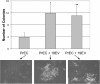
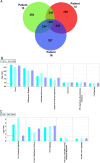
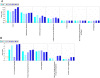
Results: We report several phenotypic changes mediated by EVs isolated from non-malignant and malignant prostate cells as well as patient biopsied prostate tumor samples. EVs can reverse the resistance of prostate cancer cells to camptothecin EVs isolated from non-malignant PrECs (Prostate Epithelial Cells) can reverse soft agar colony formation of malignant DU145 cells, with the reciprocal effect observed. Isolation of EVs from 2 Gleason grade 8 prostate cancer patients significantly induced soft agar colony formation of non-malignant PrECs. We have identified proteins via antibody and Mass spectrometry analysis that may be responsible for the phenotypic changes. Mass spectrometry analysis of protein lysates using ProteoIQ revealed protein candidates associated with gene ontology annotations that may be responsible for this phenotypic change. Ingenuity Pathway Analysis was used to identify statistically relevant canonical pathways and functions associated the protein IDs and expression values obtained using ProteoIQ. Western blot analysis confirmed the increase of 14-3-3 zeta, pRKIP and prohibitin protein levels in PrEC cells co-cultured with patient EVs. 14-3-3 proteins were also found as common proteins of 3 other Gleason grade 8 patients.
Conclusion: Our study provides a rational basis to further investigate putative proteins, such as 14-3-3 and prohibitin and genetic factors that may be responsible for phenotypic changes that are associated with prostate cancer progression.
Figures



References
-
- Bolla M, Collette L, Blank L, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C. et al.Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103–106. doi: 10.1016/S0140-6736(02)09408-4. - DOI - PubMed
-
- Hanks GE, Pajak TF, Porter A, Grignon D, Brereton H, Venkatesan V, Horwitz EM, Lawton C, Rosenthal SA, Sandler HM. et al.Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the radiation therapy oncology group protocol 92–02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. - DOI - PubMed
-
- Pilepich MV, Winter K, Lawton CA, Krisch RE, Wolkov HB, Movsas B, Hug EB, Asbell SO, Grignon D. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma–long-term results of phase III RTOG 85–31. Int J Radiat Oncol Biol Phys. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. - DOI - PubMed
-
- Pilepich MV, Winter K, John MJ, Mesic JB, Sause W, Rubin P, Lawton C, Machtay M, Grignon D. Phase III radiation therapy oncology group (RTOG) trial 86–10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/S0360-3016(01)01579-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

