Label-free in vitro toxicity and uptake assessment of citrate stabilised gold nanoparticles in three cell lines
- PMID: 24103467
- PMCID: PMC3853235
- DOI: 10.1186/1743-8977-10-50
Label-free in vitro toxicity and uptake assessment of citrate stabilised gold nanoparticles in three cell lines
Abstract
Background: Reliable in vitro toxicity testing is needed prior to the commencement of in vivo testing necessary for hazard identification and risk assessment of nanoparticles. In this study, the cytotoxicity and uptake of 14 nm and 20 nm citrate stabilised gold nanoparticles (AuNPs) in the bronchial epithelial cell line BEAS-2B, the Chinese hamster ovary cell line CHO, and the human embryonic kidney cell line HEK 293 were investigated.
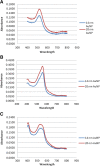
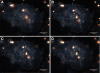
Methods: Cytotoxicity of the AuNPs was assessed via traditional XTT-, LDH-, and ATP-based assays, followed by cell impedance studies. Dark-field imaging and hyperspectral imaging were used to confirm the uptake of AuNPs into the cells.
Results: Interference of the AuNPs with the XTT- and ATP-based assays was overcome through the use of cell impedance technology. AuNPs were shown to be relatively non-toxic using this methodology; nevertheless CHO cells were the most sensitive cell type with 20 nm AuNPs having the highest toxicity. Uptake of both 14 nm and 20 nm AuNPs was observed in all cell lines in a time- and cell type-dependent manner.
Conclusions: Using the cell impedance and dark-field hyperspectral imaging technologies, it was possible to study the toxicity of AuNPs in different cell lines and show that these cells could internalize AuNPs with their subsequent intracellular aggregation. It was also possible to show that this toxicity would not correlate with the level of uptake but it would correlate with cell-type and the size of the AuNPs. Therefore, these two label-free methodologies used in this study are suitable for in vitro studies on the effects of AuNPs, and could present themselves as appropriate and valuable methodologies for future nanoparticle toxicity and uptake studies.
Figures








Similar articles
-
From the Cover: An Investigation of the Genotoxicity and Interference of Gold Nanoparticles in Commonly Used In Vitro Mutagenicity and Genotoxicity Assays.Toxicol Sci. 2017 Mar 1;156(1):149-166. doi: 10.1093/toxsci/kfw247. Toxicol Sci. 2017. PMID: 28108664
-
Cytotoxicity, intracellular localization and exocytosis of citrate capped and PEG functionalized gold nanoparticles in human hepatocyte and kidney cells.Cell Biol Toxicol. 2016 Aug;32(4):305-21. doi: 10.1007/s10565-016-9336-y. Epub 2016 May 16. Cell Biol Toxicol. 2016. PMID: 27184667
-
Uptake and cytotoxicity of citrate-coated gold nanospheres: Comparative studies on human endothelial and epithelial cells.Part Fibre Toxicol. 2012 Jul 3;9:23. doi: 10.1186/1743-8977-9-23. Part Fibre Toxicol. 2012. PMID: 22759355 Free PMC article.
-
Toxic effects and biodistribution of ultrasmall gold nanoparticles.Arch Toxicol. 2017 Sep;91(9):3011-3037. doi: 10.1007/s00204-017-2016-8. Epub 2017 Jul 12. Arch Toxicol. 2017. PMID: 28702691 Review.
-
Gold Nanoparticles (AuNPs)-Toxicity, Safety and Green Synthesis: A Critical Review.Int J Mol Sci. 2024 Apr 5;25(7):4057. doi: 10.3390/ijms25074057. Int J Mol Sci. 2024. PMID: 38612865 Free PMC article. Review.
Cited by
-
Biosynthesis of copper oxide nanoparticles mediated Annona muricata as cytotoxic and apoptosis inducer factor in breast cancer cell lines.Sci Rep. 2022 Sep 28;12(1):16165. doi: 10.1038/s41598-022-20360-y. Sci Rep. 2022. PMID: 36171339 Free PMC article.
-
Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract.Sci Rep. 2019 Mar 12;9(1):4169. doi: 10.1038/s41598-019-40816-y. Sci Rep. 2019. PMID: 30862803 Free PMC article.
-
Acetylation of Aβ42 at Lysine 16 Disrupts Amyloid Formation.ACS Chem Neurosci. 2020 Apr 15;11(8):1178-1191. doi: 10.1021/acschemneuro.0c00069. Epub 2020 Apr 2. ACS Chem Neurosci. 2020. PMID: 32207962 Free PMC article.
-
Aneuploidogenic effects and DNA oxidation induced in vitro by differently sized gold nanoparticles.Int J Nanomedicine. 2014 May 8;9:2191-204. doi: 10.2147/IJN.S58397. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24855356 Free PMC article.
-
A screening approach for the evaluation of tobacco-free 'modern oral' nicotine products using Real Time Cell Analysis.Toxicol Rep. 2021 Feb 25;8:481-488. doi: 10.1016/j.toxrep.2021.02.014. eCollection 2021. Toxicol Rep. 2021. PMID: 33718000 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

