Cyclodextrins in non-viral gene delivery
- PMID: 24103652
- PMCID: PMC7112483
- DOI: 10.1016/j.biomaterials.2013.09.061
Cyclodextrins in non-viral gene delivery
Abstract
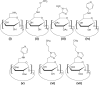
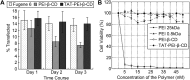
Cyclodextrins (CDs) are naturally occurring cyclic oligosaccharides. They consist of (α-1,4)-linked glucose units, and possess a basket-shaped topology with an "inner-outer" amphiphilic character. Over the years, substantial efforts have been undertaken to investigate the possible use of CDs in drug delivery and controlled drug release, yet the potential of CDs in gene delivery has received comparatively less discussion in the literature. In this article, we will first discuss the properties of CDs for gene delivery, followed by a synopsis of the use of CDs in development and modification of non-viral gene carriers. Finally, areas that are noteworthy in CD-based gene delivery will be highlighted for future research. Due to the application prospects of CDs, it is anticipated that CDs will continue to emerge as an important tool for vector development, and will play significant roles in facilitating non-viral gene delivery in the forthcoming decades.
Keywords: Cyclodextrin; Gene delivery; Non-viral vector; Polymer; Transfection.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
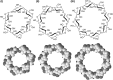
Similar articles
-
Chemistry and engineering of cyclodextrins for molecular imaging.Chem Soc Rev. 2017 Oct 16;46(20):6379-6419. doi: 10.1039/c7cs00040e. Chem Soc Rev. 2017. PMID: 28930330 Review.
-
Gastrointestinal gene delivery by cyclodextrins--in vitro quantification of extracellular barriers.Int J Pharm. 2013 Nov 18;456(2):390-9. doi: 10.1016/j.ijpharm.2013.08.073. Epub 2013 Sep 7. Int J Pharm. 2013. PMID: 24016741
-
Cyclodextrin-based supramolecular architectures: syntheses, structures, and applications for drug and gene delivery.Adv Drug Deliv Rev. 2008 Jun 10;60(9):1000-17. doi: 10.1016/j.addr.2008.02.011. Epub 2008 Mar 4. Adv Drug Deliv Rev. 2008. PMID: 18413280 Review.
-
Characterisation of cationic amphiphilic cyclodextrins for neuronal delivery of siRNA: effect of reversing primary and secondary face modifications.Eur J Pharm Sci. 2012 Dec 18;47(5):896-903. doi: 10.1016/j.ejps.2012.08.020. Epub 2012 Sep 25. Eur J Pharm Sci. 2012. PMID: 23022516
-
Cyclodextrin-membrane interaction in drug delivery and membrane structure maintenance.Int J Pharm. 2019 Jun 10;564:59-76. doi: 10.1016/j.ijpharm.2019.03.063. Epub 2019 Apr 5. Int J Pharm. 2019. PMID: 30959238 Review.
Cited by
-
Metallohelix vectors for efficient gene delivery via cationic DNA nanoparticles.Nucleic Acids Res. 2022 Jan 25;50(2):674-683. doi: 10.1093/nar/gkab1277. Nucleic Acids Res. 2022. PMID: 35018455 Free PMC article.
-
Tetraethylenepentamine-Coated β Cyclodextrin Nanoparticles for Dual DNA and siRNA Delivery.Pharmaceutics. 2022 Apr 23;14(5):921. doi: 10.3390/pharmaceutics14050921. Pharmaceutics. 2022. PMID: 35631507 Free PMC article.
-
Anti-inflammatory effects of cyclodextrin nanoparticles enable macrophage repolarization and reduce inflammation.Discov Nano. 2024 Dec 21;19(1):211. doi: 10.1186/s11671-024-04175-6. Discov Nano. 2024. PMID: 39707045 Free PMC article.
-
Binding Thermodynamics and Kinetics Calculations Using Chemical Host and Guest: A Comprehensive Picture of Molecular Recognition.J Chem Theory Comput. 2018 Jan 9;14(1):303-318. doi: 10.1021/acs.jctc.7b00899. Epub 2017 Dec 14. J Chem Theory Comput. 2018. PMID: 29149564 Free PMC article.
-
Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics.Pharmaceutics. 2025 Feb 22;17(3):288. doi: 10.3390/pharmaceutics17030288. Pharmaceutics. 2025. PMID: 40142952 Free PMC article. Review.
References
-
- Del Valle E.M.M. Cyclodextrins and their uses: a review. Process Biochem. 2004;39:1033–1046.
-
- Villiers A. Sur la fermentation de la fécule par l'action du ferment butyrique. Compt Rendus Acad Sci. 1891;112:536–538.
-
- Freudenberg K., Meyer-Delius M. Über die Schardinger–Dextrine aus Stärke. Ber dtsch Chem Ges A/B. 1938;71:1596–1600.
-
- Freudenberg K., Schaaf E., Dumpert G., Ploetz T. Neue Ansichten über die Stärke. Naturwissenschaften. 1939;51:850–853.
-
- Cramer F. Springer-Verlag; Berlin: 1954. Einschlussverbindungen.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

