Interleukin-3 plays dual roles in osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process
- PMID: 24103757
- PMCID: PMC3856188
- DOI: 10.1016/j.bbrc.2013.09.098
Interleukin-3 plays dual roles in osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process
Abstract
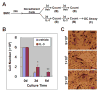
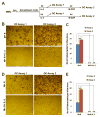
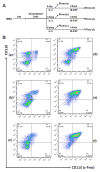
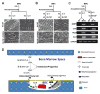
Interleukin (IL)-3, a multilineage hematopoietic growth factor, is implicated in the regulation of osteoclastogenesis. However, the role of IL-3 in osteoclastogenesis remains controversial; whereas early studies showed that IL-3 stimulates osteoclastogenesis, recent investigations demonstrated that IL-3 inhibits osteoclast formation. The objective of this work is to further address the role of IL-3 in osteoclastogenesis. We found that IL-3 treatment of bone marrow cells generated a population of cells capable of differentiating into osteoclasts in tissue culture dishes in response to the stimulation of the monocyte/macrophage-colony stimulating factor (M-CSF) and the receptor activator of nuclear factor kappa B ligand (RANKL). The IL-3-dependent hematopoietic cells were able to further proliferate and differentiate in response to M-CSF stimulation and the resulting cells were also capable of forming osteoclasts with M-CSF and RANKL treatment. Interestingly, IL-3 inhibits M-CSF-/RANKL-induced differentiation of the IL-3-dependent hematopoietic cells into osteoclasts. The flow cytometry analysis indicates that while IL-3 treatment of bone marrow cells slightly affected the percentage of osteoclast precursors in the surviving populations, it considerably increased the percentage of osteoclast precursors in the populations after subsequent M-CSF treatment. Moreover, osteoclasts derived from IL-3-dependent hematopoietic cells were fully functional. Thus, we conclude that IL-3 plays dual roles in osteoclastogenesis by promoting the development of osteoclast progenitors but inhibiting the osteoclastogenic process. These findings provide a better understanding of the role of IL-3 in osteoclastogenesis.
Keywords: APC; BMC; BRC; CMP; Car2; Ctsk; FBS; GAPDH; GM-CSF; GMP; HSC; IL-3; IL-6; Interleukin-3; M-CSF; MMP9; MSC; Osteoclast precursor; Osteoclast progenitor; Osteoclastogenesis; PBS; PE; RANKL; RT-PCR; SCF; SEM; TRAP; allophycocyanin; bone marrow cells; bone remodeling compartment; carbonic anhydrase 2; cathepsin K; common myeloid progenitors; fetal bovine serum; glyceraldehyde 3-phosphate dehydrogenase; granulocyte/macrophage colony stimulating factor; granulocyte/macrophage progenitors; hematopoietic stem cells; interleukin 3; interleukin 6; matrix metalloproteinase 9; mesenchymal stem cells; monocyte/macrophage-colony stimulating factor; phosphate-buffered buffers; phycoerythrin; receptor activator of nuclear factor kappa B ligand; reverse transcription-polymerase chain reaction; scanning electron microscopy; stem cell factor; tartrate resistant acid phosphatase; α-MEM; α-minimal essential medium.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Presence of osteoclast precursors in colonies cloned in the presence of hematopoietic colony-stimulating factors.Exp Hematol. 2001 Jan;29(1):68-76. doi: 10.1016/s0301-472x(00)00626-3. Exp Hematol. 2001. PMID: 11164107
-
The generation of highly enriched osteoclast-lineage cell populations.Bone. 2002 Jan;30(1):164-70. doi: 10.1016/s8756-3282(01)00654-8. Bone. 2002. PMID: 11792580
-
NF-kappaB p50 and p52 expression is not required for RANK-expressing osteoclast progenitor formation but is essential for RANK- and cytokine-mediated osteoclastogenesis.J Bone Miner Res. 2002 Jul;17(7):1200-10. doi: 10.1359/jbmr.2002.17.7.1200. J Bone Miner Res. 2002. PMID: 12096833
-
The molecular basis of osteoclast differentiation and activation.Novartis Found Symp. 2001;232:235-47; discussion 247-50. doi: 10.1002/0470846658.ch16. Novartis Found Symp. 2001. PMID: 11277084 Review.
-
Epigenetic and transcriptional regulation of osteoclast differentiation.Bone. 2020 Sep;138:115471. doi: 10.1016/j.bone.2020.115471. Epub 2020 Jun 8. Bone. 2020. PMID: 32526404 Review.
Cited by
-
Notch signaling promotes osteoclast maturation and resorptive activity.J Cell Biochem. 2015 Nov;116(11):2598-609. doi: 10.1002/jcb.25205. J Cell Biochem. 2015. PMID: 25914241 Free PMC article.
-
Assembling the Puzzle Pieces. Insights for in Vitro Bone Remodeling.Stem Cell Rev Rep. 2023 Aug;19(6):1635-1658. doi: 10.1007/s12015-023-10558-6. Epub 2023 May 19. Stem Cell Rev Rep. 2023. PMID: 37204634 Review.
-
Gene expression responses to Zika virus infection in peripheral blood mononuclear cells from pregnant and non-pregnant women.Microbiologyopen. 2020 Dec;9(12):e1134. doi: 10.1002/mbo3.1134. Epub 2020 Nov 19. Microbiologyopen. 2020. PMID: 33211409 Free PMC article.
References
-
- Feng X. Regulatory roles and molecular signaling of TNF family members in osteoclasts. Gene. 2005;350:1–13. - PubMed
-
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

