Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease
- PMID: 24106153
- PMCID: PMC4474604
- DOI: 10.1002/pbc.24772
Clofarabine salvage therapy in refractory multifocal histiocytic disorders, including Langerhans cell histiocytosis, juvenile xanthogranuloma and Rosai-Dorfman disease
Abstract
Background: Existing therapies for recurrent or refractory histiocytoses, including Langerhans cell histiocytosis (LCH), juvenile xanthogranuloma (JXG), and Rosai-Dorfman disease (RDD), have limited effectiveness. We report our experience with using clofarabine as therapy in children with recurrent or refractory histiocytic disorders, including LCH (11 patients), systemic JXG (4 patients), and RDD (3 patients).
Methods: Patients treated with clofarabine for LCH, JXG, or RDD by Texas Children's Hospital physicians or collaborators between May 2011 and January 2013 were reviewed for response and toxicity.
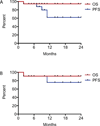
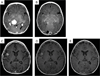
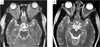
Results: Patients were treated with a median of three chemotherapeutic regimens prior to clofarabine. Clofarabine was typically administered at 25 mg/m(2) /day for 5 days. Cycles were administered every 28 days for a median of six cycles (range: 2-8 cycles). Seventeen of 18 patients are alive. All surviving patients showed demonstrable improvement after two to four cycles of therapy, with 11 (61%) complete responses, 4 (22%) partial responses, and 2 patients still receiving therapy. Five patients experienced disease recurrence, but three of these subsequently achieved complete remission. All patients with JXG and RDD had complete or partial response at conclusion of therapy. Side effects included neutropenia in all patients. Recurring but sporadic toxicities included prolonged neutropenia, severe vomiting, and bacterial infections.
Conclusion: Clofarabine has activity against LCH, JXG, and RDD in heavily pretreated patients, but prospective multi-center trials are warranted to determine long-term efficacy, optimal dosing, and late toxicity of clofarabine in this population.
Keywords: Langerhans cell histiocytosis; Rosai-Dorfman disease; clofarabine; histiocytosis; juvenile xanthogranuloma.
© 2013 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest statement: the authors have no conflicts of interest.
Figures
References
-
- Grois N, Fahrner B, Arceci RJ, et al. Central nervous system disease in Langerhans cell histiocytosis. J Pediatr. 2010;156(6):873–881. 881 e871. - PubMed
-
- Allen CE, McClain KL. Langerhans cell histiocytosis: a review of past, current and future therapies. Drugs Today (Barc) 2007;43(9):627–643. - PubMed
-
- Kudo K, Ohga S, Morimoto A, et al. Improved outcome of refractory Langerhans cell histiocytosis in children with hematopoietic stem cell transplantation in Japan. Bone Marrow Transplant. 2010;45(5):901–906. - PubMed
-
- Gadner H, Minkov M, Grois N, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121(25):5006–5014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

