Gene therapy on the move
- PMID: 24106209
- PMCID: PMC3840483
- DOI: 10.1002/emmm.201202287
Gene therapy on the move
Abstract
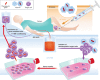
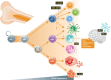
The first gene therapy clinical trials were initiated more than two decades ago. In the early days, gene therapy shared the fate of many experimental medicine approaches and was impeded by the occurrence of severe side effects in a few treated patients. The understanding of the molecular and cellular mechanisms leading to treatment- and/or vector-associated setbacks has resulted in the development of highly sophisticated gene transfer tools with improved safety and therapeutic efficacy. Employing these advanced tools, a series of Phase I/II trials were started in the past few years with excellent clinical results and no side effects reported so far. Moreover, highly efficient gene targeting strategies and site-directed gene editing technologies have been developed and applied clinically. With more than 1900 clinical trials to date, gene therapy has moved from a vision to clinical reality. This review focuses on the application of gene therapy for the correction of inherited diseases, the limitations and drawbacks encountered in some of the early clinical trials and the revival of gene therapy as a powerful treatment option for the correction of monogenic disorders.
Keywords: clinical trials; iPS; monogenic disorders; stem cell therapy; viral vectors.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures


References
-
- Aiuti A, Bacchetta R, Seger R, Villa A, Cavazzana-Calvo M. Gene therapy for primary immunodeficiencies: Part 2. Curr Opin Immunol. 2012;24:585–591. - PubMed
-
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, Scaramuzza S, Andolfi G, Mirolo M, Brigida I, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. - PubMed
-
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

