Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice
- PMID: 24106222
- PMCID: PMC3840485
- DOI: 10.1002/emmm.201302857
Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice
Abstract
A major complication of factor replacement therapy for haemophilia is the development of anti-factor neutralizing antibodies (inhibitors). Here we show that liver gene therapy by lentiviral vectors (LVs) expressing factor IX (FIX) strongly reduces pre-existing anti-FIX antibodies and eradicates FIX inhibitors in haemophilia B mice. Concomitantly, plasma FIX levels and clotting activity rose to 50-100% of normal. The treatment was effective in 75% of treated mice. FIX-specific plasma cells (PCs) and memory B cells were reduced, likely because of memory B-cell depletion in response to constant exposure to high doses of FIX. Regulatory T cells displaying FIX-specific suppressive capacity were induced in gene therapy treated mice and controlled FIX-specific T helper cells. Gene therapy proved safer than a regimen mimicking immune tolerance induction (ITI) by repeated high-dose FIX protein administration, which induced severe anaphylactoid reactions in inhibitors-positive haemophilia B mice. Liver gene therapy can thus reverse pre-existing immunity, induce active tolerance to FIX and establish sustained FIX activity at therapeutic levels. These data position gene therapy as an attractive treatment option for inhibitors-positive haemophilic patients.
Keywords: gene therapy; haemophilia; immune tolerance.
© 2013 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
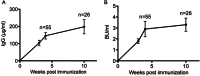
Anti-FIX IgG Abs measured by ELISA in plasma samples collected from mice at the indicated times after FIX immunization. Data are presented as mean ± standard error of the mean (SEM).
FIX inhibitors measured by Bethesda Assay in plasma samples collected from mice at the indicated times after FIX immunization. Data are presented as mean ± standard error of the mean (SEM).
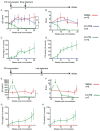
A,E. Anti-FIX IgG Abs measured by ELISA in plasma samples collected from mice at the indicated times after FIX immunization.
B,F. FIX inhibitors measured by Bethesda Assay in plasma samples collected from mice at the indicated times after FIX immunization.
C,G. FIX expression measured by ELISA in plasma samples collected from mice at the indicated times after FIX immunization.
D,H. FIX activity measured by aPTT in plasma samples collected from mice at the indicated times after FIX immunization.
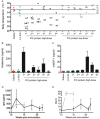
A. Body temperature measured 30–45 minutes after saline (red circles, n = 5), LV-FIX (green circles, n = 5) or after each recombinant high-dose (black circles, n = 5) or low-dose (grey circles, n = 5) FIX protein administration. Data from single mice are plotted. The mean and SD (35.2 ± 1.9) of repeated measurement of body temperature in 6 naïve haemophilia B mice is shown. The allergy score is reported for each mouse when not 0. Allergy score was performed according to (Li et al, 2003): 0 = no symptoms; 1 = scratching and rubbing around the snout and head; 2 = puffiness around the eyes and snout, diarrhea, pilarerecti, reduced activity, and/or decreased activity with increased respiratory rate; 3 = wheezing, labored respiration, cyanosis around the mouth and the tail; 4 = no activity after prodding, or tremor and convulsion; 5 = death.
B,C. Histamine and mouse mast-cell protease 1 (mMCP-1) measured by ELISA in plasma samples collected 30–45 min after saline (red bar, n = 5), LV-FIX (green bar, n = 5) or after each recombinant high-dose (black bars, n = 5) FIX protein administration. Data are presented as mean ± SEM.
D. Anti-FIX IgG Abs measured by ELISA in plasma samples collected from mice treated with i.v. recombinant FIX protein injections at high dose (black line, n = 9) or low dose (grey line, n = 5) at the indicated times after FIX immunization. Data are presented as mean ± SEM.
E. FIX inhibitors measured by Bethesda Assay in plasma samples collected from mice treated with i.v. recombinant FIX protein injections at high dose (black line, n = 9) or low dose (grey line, n = 5) at the indicated times after FIX immunization. Data are presented as mean ± SEM.
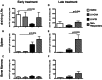
A–C. FIX-specific IgG secreting plasma cells (PCs) out of 106 total B or bone marrow cells were enumerated by elispot assay in the draining LNs, Spleen and Bone Marrow (as indicated) isolated from in inhibitors-positive haemophilia B mice 16 weeks after early treatment.
D–F. FIX-specific IgG secreting plasma cells (PCs) out of 106 total B or bone marrow cells were enumerated by elispot assay in the draining LNs, Spleen and Bone Marrow (as indicated) isolated from in inhibitors-positive haemophilia B mice 12 weeks after late treatment.

A. OVA-specific IgG-secreting PCs, which derive from activated BMEM, were counted by elispotat the end of the Ag exposure. Data are presented as mean ± SEM.
B. FIX-specific IgG-secreting PCs, which derive from activated BMEM, were counted by elispotat the end of the Ag exposure. Data are presented as mean ± SEM.
C–D. The frequency of FIX-specific BMEM (CD19+B220+IgD−IgM−FIX+APC) was evaluated by FACS in LN cells harvested at 12 weeks after late treatment (n = 1 naïve haemoB as control; n = 3 Saline-treated; n = 4 LV-FIX treated). Data are presented as mean ± SEM. A representative dot plot for each group is shown.

FIX-driven T-cell-proliferative response was measured stimulating B cells-depleted (B220−) splenocytes isolated from inhibitors-positive haemophilia B mice, 16 weeks after the early treatment.
FIX-driven T-cell-proliferative response was measured stimulating B cells-depleted (B220−) splenocytes isolated from inhibitors-positive haemophilia B mice, 12 weeks after the late treatment.
Proliferation data are presented as mean of Stimulation Index (SI) ± SEM for each experimental group (2 independent experiments for each treatment protocol), excluding from LV-FIX treated mice, those defined as non-responders (NR, detectable BU/ml and/or FIX activity <1% of normal).
Regulatory T cells (CD4+CD25+ Tregs) and conventional T cells (CD4+CD25−Tconv) were isolated from inhibitors-positive haemophilia B mice 2–3 weeks after LV-FIX gene therapy (n = 3), at a time in which the anti-FIX Abs were lower than saline-injected controls (arrow). LV-FIX or saline were administered 4 weeks after immunization (line).
LN cells derived from OVA-immunized mice were stimulated with the relevant Ag alone or in co-culture with Tregs and Tconv (ratio 1:0.5) to define their suppressive capacity. Data are presented as mean SI ± range for each culture condition (2 independent experiments).
LN cells derived from FIX-immunized mice were stimulated with the relevant Ag alone or in co-culture with Tregs and Tconv (ratio 1:0.5) to define their suppressive capacity. Data are presented as mean SI ± range for each culture condition (2 independent experiments).
References
-
- Astermark J, Lacroix-Desmazes S, Reding M. Inhibitor development. Haemophilia: The official journal of the World Federation of. Hemophilia. 2008;14:36–42. - PubMed
-
- Astermark J, Santagostino E, Keith Hoots W. Clinical issues in inhibitors. Haemophilia. 2010;16:54–60. - PubMed
-
- Benson G, Auerswald Gn, Elezovic I, Lambert T, Ljung R, Morfini M, Remor E, Salek S. Immune tolerance induction in patients with severe hemophilia with inhibitors: Expert panel views and recommendations for clinical practice. Eur J Haematol. 2012;88:371–379. - PubMed
-
- Berntorp E, Shapiro A. Modern haemophilia care. Lancet. 2012;379:1447–1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

