Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis
- PMID: 24106267
- PMCID: PMC3837143
- DOI: 10.1074/jbc.M113.518019
Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis
Abstract
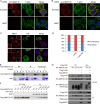
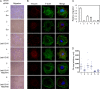
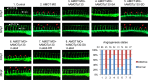
The Hippo tumor suppressor pathway plays important roles in organ size control through Lats1/2 mediated phosphorylation of the YAP/TAZ transcription co-activators. However, YAP/TAZ independent functions of the Hippo pathway are largely unknown. Here we report a novel role of the Hippo pathway in angiogenesis. Angiomotin p130 isoform (AMOTp130) is phosphorylated on a conserved HXRXXS motif by Lats1/2 downstream of GPCR signaling. Phosphorylation disrupts AMOT interaction with F-actin and correlates with reduced F-actin stress fibers and focal adhesions. Furthermore, phosphorylation of AMOT by Lats1/2 inhibits endothelial cell migration in vitro and angiogenesis in zebrafish embryos in vivo. Thus AMOT is a direct substrate of Lats1/2 mediating functions of the Hippo pathway in endothelial cell migration and angiogenesis.
Keywords: AMOT; Actin; Angiogenesis; Cell Migration; Lats; Protein Kinase; Protein Kinases; Protein Phosphorylation; the Hippo Pathway.
Figures





References
-
- Zhou D., Conrad C., Xia F., Park J. S., Payer B., Yin Y., Lauwers G. Y., Thasler W., Lee J. T., Avruch J., Bardeesy N. (2009) Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16, 425–438 - PMC - PubMed
-
- Camargo F. D., Gokhale S., Johnnidis J. B., Fu D., Bell G. W., Jaenisch R., Brummelkamp T. R. (2007) YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 - PubMed
-
- Wu S., Huang J., Dong J., Pan D. (2003) hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114, 445–456 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

